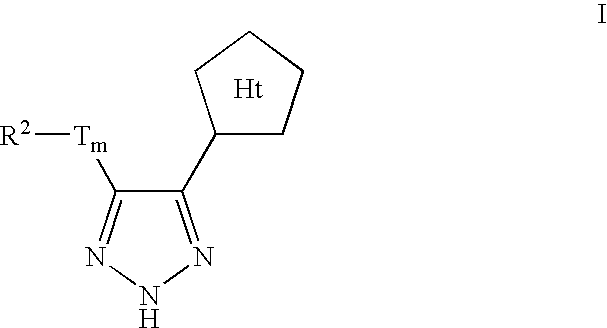
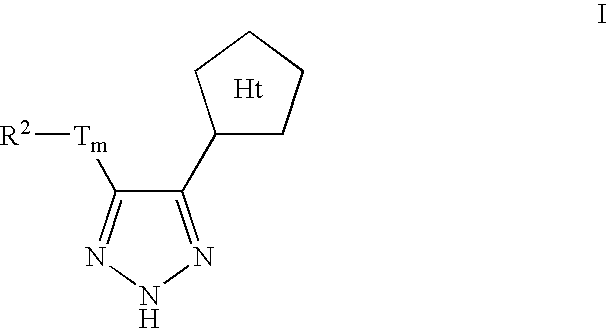
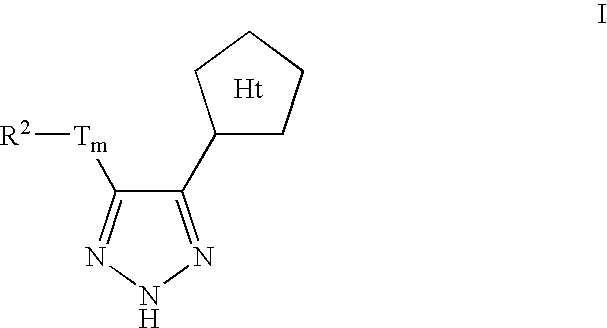
Triazole-derived kinase inhibitors and uses thereof
a kinase inhibitor and triazole technology, applied in the field of medical chemistry, can solve the problems of insufficient treatment options and unclear whether these compounds have the appropriate pharmacological profiles to be therapeutically useful, and achieve the effect of inhibiting the activity of the enzyme and facilitating the treatment or prophylaxis of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ERK Inhibition Assay
[0132] Compounds are assayed for the inhibition of ERK2 by a spectrophotometric coupled-enzyme assay (Fox et al (1998) Protein Sci 7, 2249). In this assay, a fixed concentration of activated ERK2 (10 nM) is incubated with various concentrations of the compound in DMSO (2.5%) for 10 min. at 30° C. in 0.1 M HEPES buffer, pH 7.5, containing 10 mM MgCl2, 2.5 mM phosphoenolpyruvate, 200 μM NADH, 150 μg / mL pyruvate kinase, 50 μg / mL lactate dehydrogenase, and 200 μM erktide peptide. The reaction is initiated by the addition of 65 μM ATP. The rate of decrease of absorbance at 340 nm is monitored, which indicates the extent of uninhibited enzyme present in the assay. The IC50 is evaluated from the rate data as a function of inhibitor concentration.
example 2
ERK Inhibition Cell Proliferation Assay
[0133] Compounds may be assayed for the inhibition of ERK2 by a cell proliferation assay. In this assay, a complete media is prepared by adding 10% fetal bovine serum and penicillin / streptomycin solution to RPMI 1640 medium (JRH Biosciences). Colon cancer cells (HT-29 cell line) are added to each of 84 wells of a 96 well plate at a seeding density of 10,000 cells / well / 150 μL. The cells are allowed to attach to the plate by incubating at 37° C. for 2 hours. A solution of test compound is prepared in complete media by serial dilution to obtain the following concentrations: 20 μM, 6.7 μM, 2.2 μM, 0.74 μM, 0.25 μM, and 0.08 μM. The test compound solution (50 μL) is added to each of 72 cell-containing wells. To the 12 remaining cell-containing wells, only complete media (200 μL) is added to form a control group in order to measure maximal proliferation. To the remaining 12 empty wells, complete media is added to form a vehicle control group in ord...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com