Pharmaceutical emulsions for retroviral protease inhibitors
a protease inhibitor and emulsion technology, applied in the field of pharmaceutical compositions, can solve the problems of high trough level in the clinic, limited synthetic surfactant catalog, and potential for sustained releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0050]
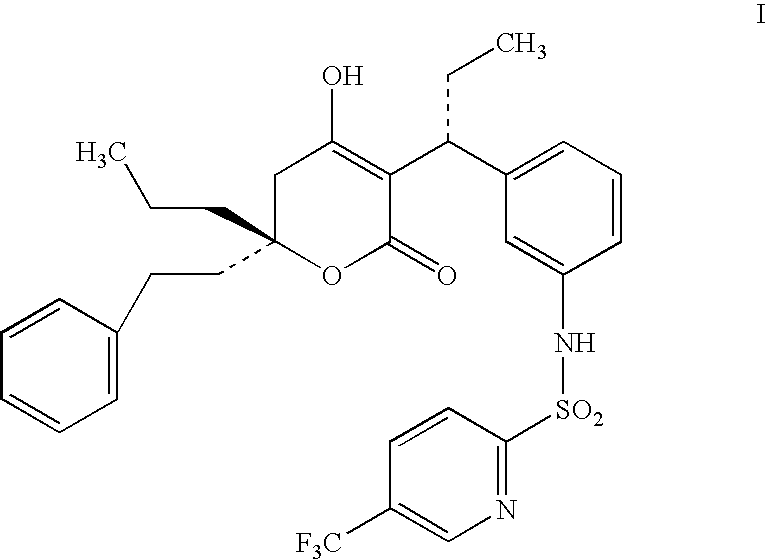
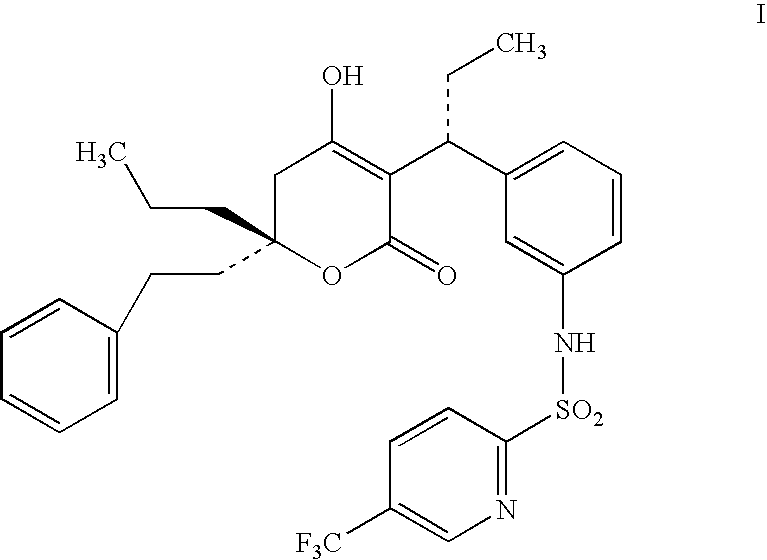
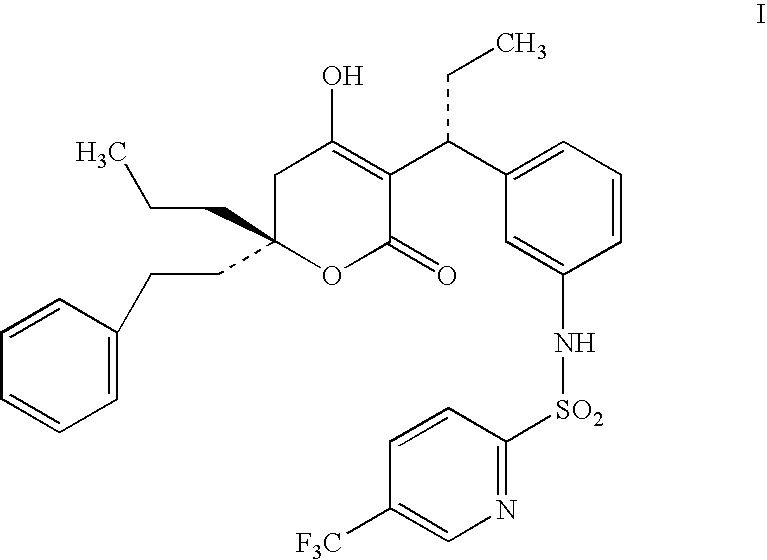
ComponentWeight (mg)Compound of Formula I60Miglyol 812200Propylene Glycol100Lecithin20Na Deoxycholate0.5glycerine24Methyl paraben1.8Propyl paraben0.2Waterq.s.
example 2
[0051]
ComponentWeight (mg)Compound of Formula I60GDO / GMO (8:2)200Propylene Glycol100Lecithin20Na Deoxycholate0.5glycerine2.4Methyl paraben1.8Propyl paraben0.2Waterq.s.
example 3
[0052]
ComponentWeight (mg)Compound of Formula I60Miglyol 812200Propylene Glycol100Lecithin20Na Deoxycholate0.5glycerine24Methyl paraben1.8Propyl paraben0.2Waterq.s.
[0053] The examples 1 and 3 are the same formulation but different particle sizes
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
| chain length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com