Chimeric anti-VEGF-D antibodies and humanized anti-VEGF-D antibodies and methods of using same
a technology of vegf-d and chimeric antivegfd, which is applied in the field of humanized anti-vegf-d antibodies and chimeric antivegf-d antibodies, can solve the problems of embryonic lethality around midgestation, little is known about the mechanisms leading to metastasis via bloodstream or lymphatics, and the ligand for tie has not yet been identified, so as to reduce unwanted angiogenesis, improve the state of the animal, and reduce tumor cell survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of Murine / Human Heavy and Light V-Region Genes
[0288] The following procedures pertain to construction of a chimeric antibody wherein variable regions from a mouse anti-VEGF-D antibody are assembled into human constant region to create a chimeric, humanized antibody.
[0289] The monoclonal antibody used for generating a chimeric antibody was the mouse anti-VEGF-D antibody produced by the hybridoma VD1 / 4A5 [deposited in the American Type Culture Collection, 10801 University Boulevard, Manassas, Va. 20110-2209, on Apr. 16, 1999 (ATCC No. HB-12698]. The deposit was made under the requirements of the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure. Production of the VD1 / 4A5 antibody is described in U.S. Pat. No. 6,383,484 (Achen et al.), incorporated herein by reference.
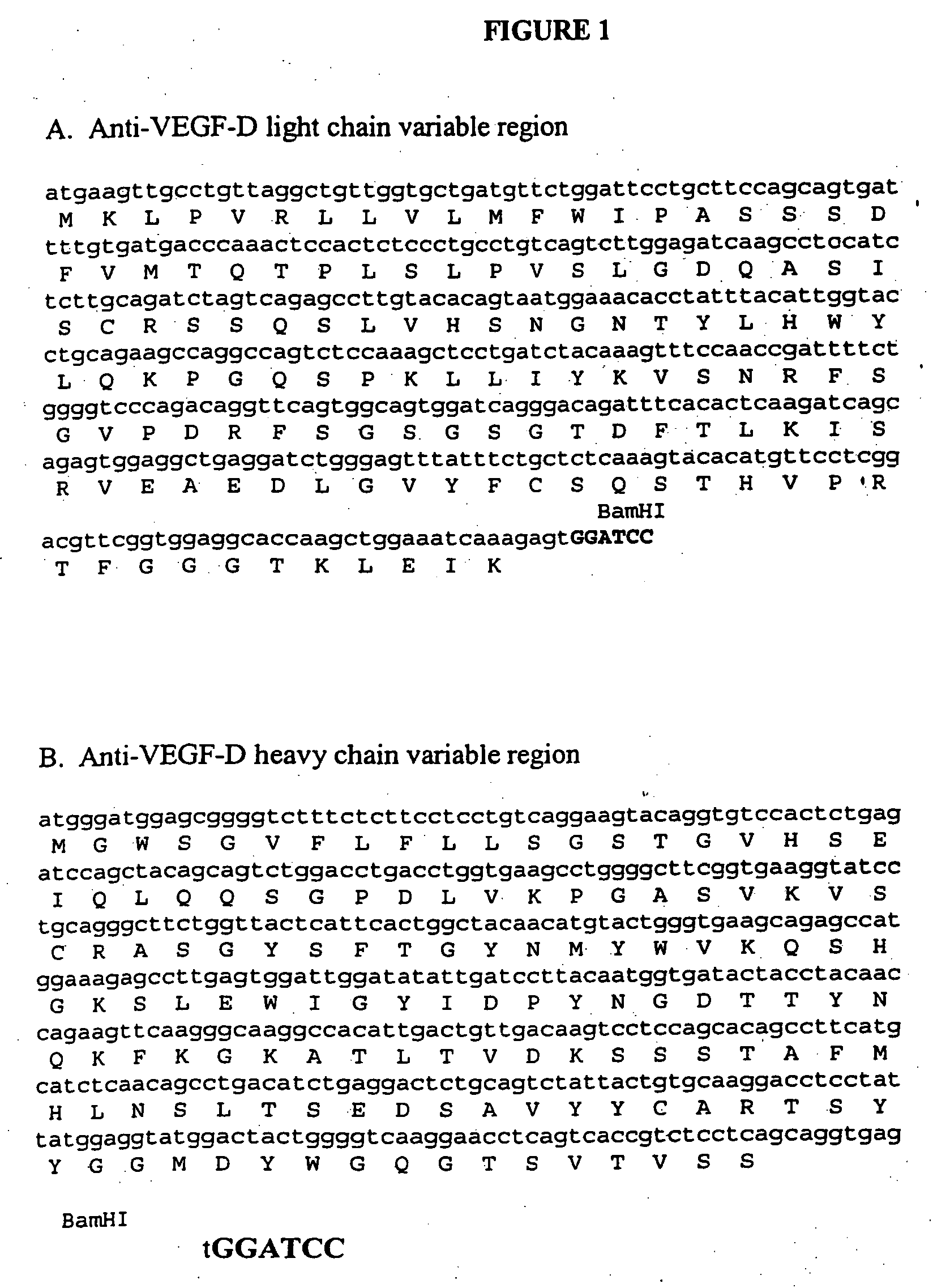
[0290] In order to begin constructing a chimeric VEGF-D antibody, it was first necessary to clone and sequence the light chain and heavy chai...
example 2
Construction of Human IgG1 VEGF-D Expression Vectors
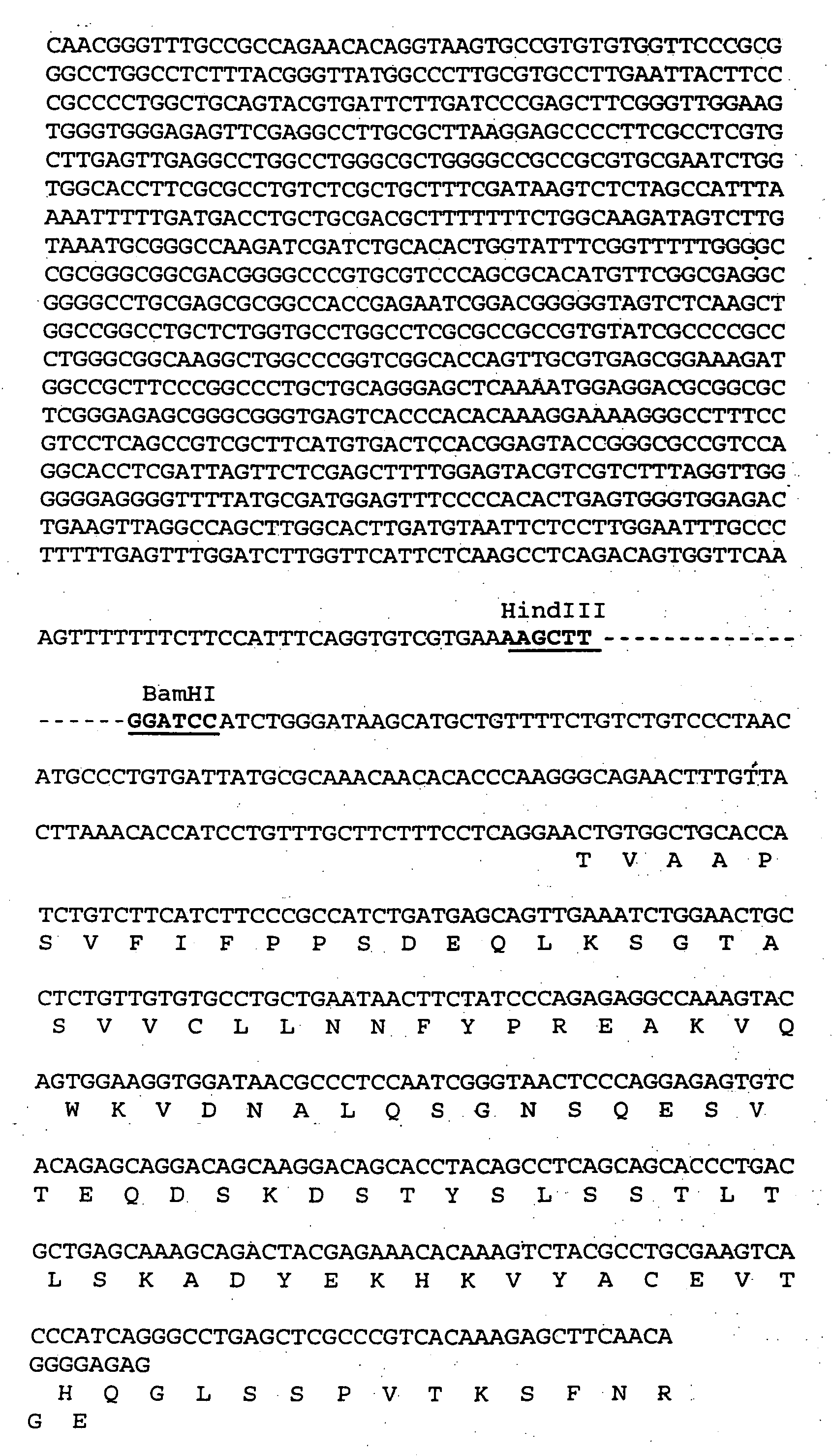
[0296] Next, an expression vector comprising the mouse anti-VEGF-D antibody variable regions and human immunoglobulin constant region was assembled.
[0297] PCR was used to modify the ends of the anti-VEGF-D light chain (SEQ ID NO: 36 and 37) and heavy chain (SEQ ID NO: 38 and 39) sequences listed in FIGS. 1A and 1B. The PCR primers used to modify the 5′ and 3′ sequences flanking the cDNA sequences are set out in Table 5. The 5′ end of the cDNA flanking sequences were modified by adding a HindIII restriction site followed by a standard Kozak sequence (GCCGCCACC, SEQ ID NO: 40), (Kozak, Nucleic Acids Res 15: 8125-48, 1987). All cDNA sequences was modified at the 3′ end to include a splice donor site and an intron after the last amino acid of the variable domain. A BamHI restriction site was included in the intron for insertion into the expression vector. Thus, each heavy and light chain construct comprised a HindIII restriction site...
example 3
Chimeric Antibody Expression and Initial Characterization of Binding Activity
[0298] The chimeric anti-VEGF-D antibody construct was expressed by transient gene expression in HEK293 cells according to the method published by Meissner and colleagues (Meissner et al., Biotechnol Bioeng. 75:197-203, 2001).
[0299] Modified HEK 293-EBNA cells (Meissner et al., supra) were cultured in Ex-Cell V Pro media (Lexena, USA). Transfection of suspension adapted HEK293-EBNA cells was carried out in DMEM / F12 medium supplemented with 29 mM sodium bicarbonate, 10 mM HEPES, 2.5 mg / L human transferrin, 2.5 mg / L insulin, 0.1 mM diethanolamine, 0.1 mM L-proline, and 1% FCS (hereafter DMEM-based medium). Prior to transfection, cells were expanded in 0.5 L spinner flasks in 293G medium (Bio-Whittaker, Walkersville, Md., USA) supplemented with 1% FCS. For transfection, cells were centrifuged in 250 ml bottles for 5 minutes at 400 g and 200 ml spinner flasks were inoculated with 1×106 cells / ml freshly resusp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com