Solid sandstone dissolver
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
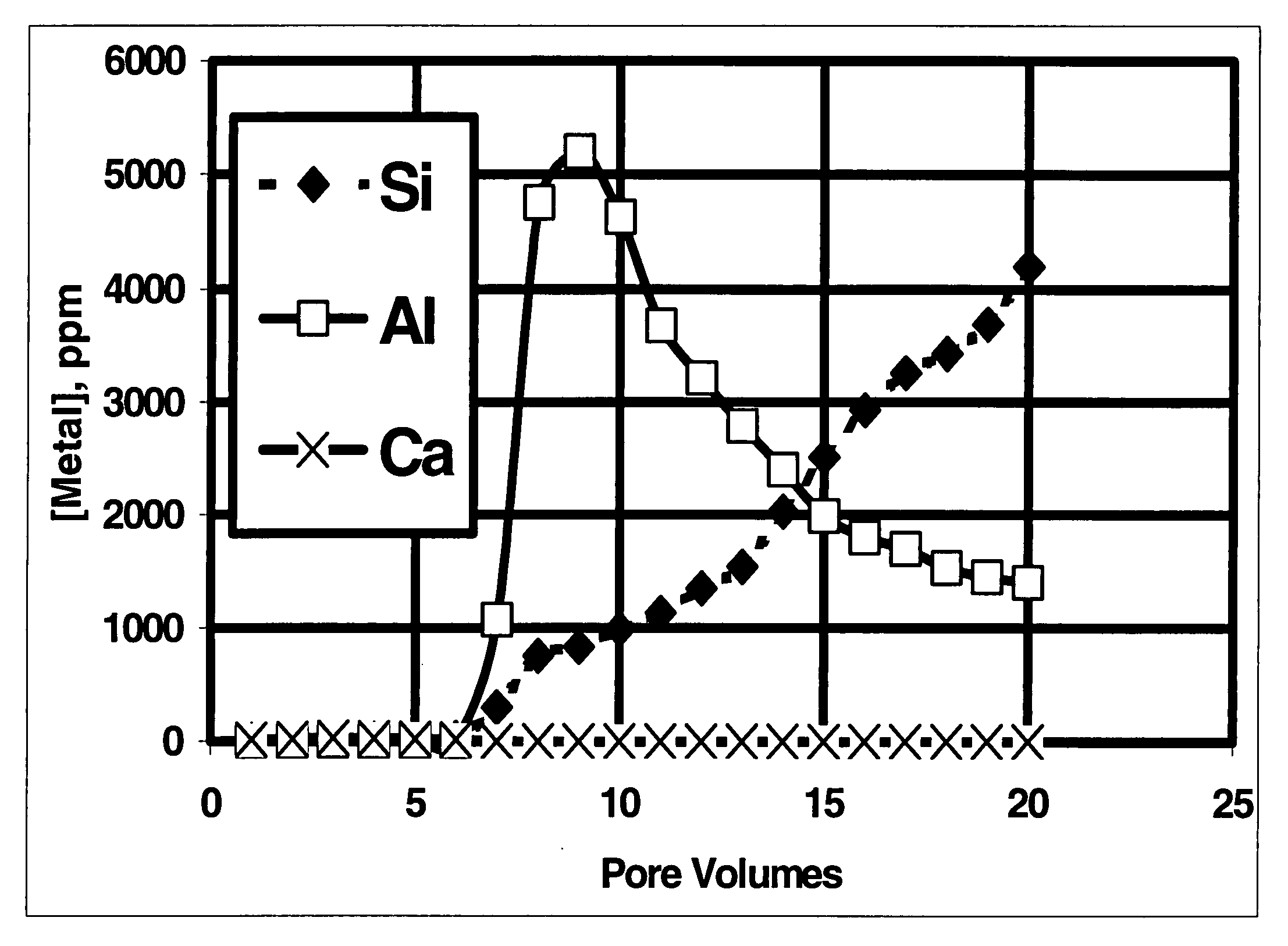
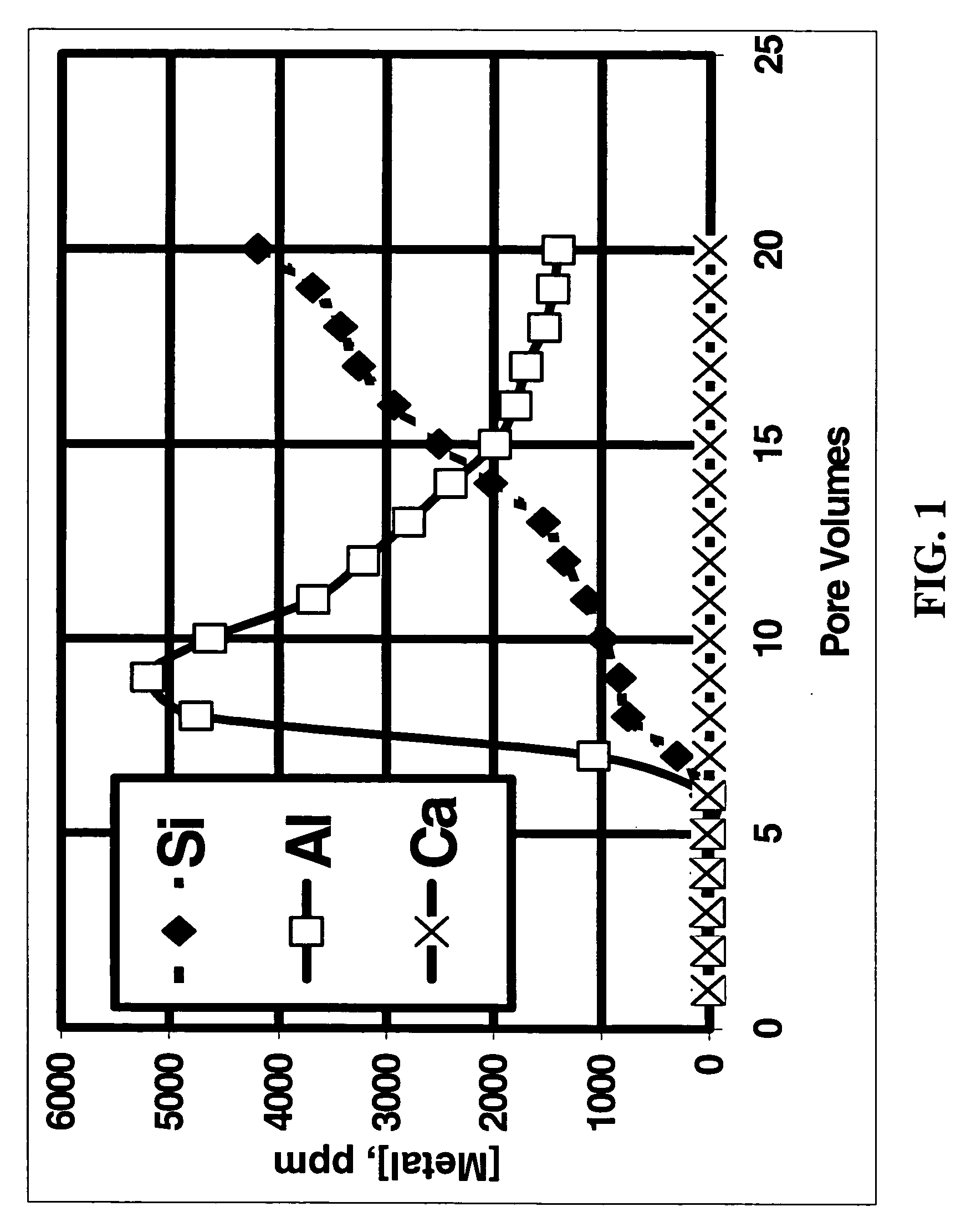
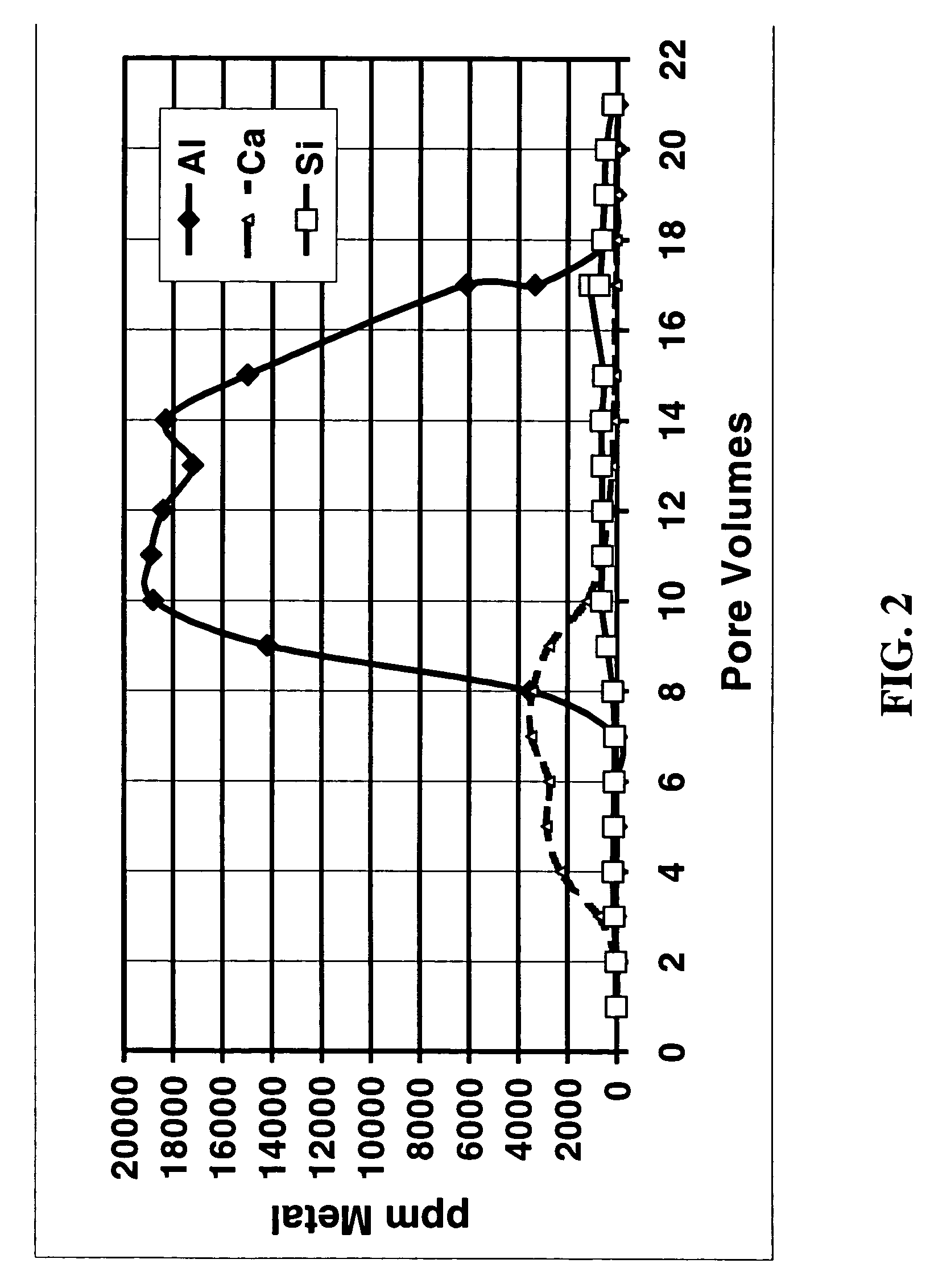
[0047] To simulate the effects of acid fracturing with a slurry of polylactic acid and ammonium bifluoride, a fluid containing 10% lactic acid (the hydrolysis / dissolution product of polylactic acid) and 4% ammonium bifluoride was injected into a 2.5 cm by 15.2 cm Berea sandstone core at 350° F. (177° C.) at a flow rate of 2.5 ml / min with a confining pressure of 6.9 MPa and a back pressure of 10.3 MPa. FIG. 1 shows a plot of different metal concentrations measured in the effluent fluid as a function of the total fluid volume pumped during a single core flow experiment. The fluid was collected at the outlet of the core flow equipment and was analyzed by ICP. The steady increase in Si concentration is a clear indication that, in combination with a fluoride-containing fluid, the polylactic acid hydrolysis product dissolved a significant amount of silicates from the sandstone. For comparison, FIG. 2 shows the results when a fluid that was 10% acetic acid in 9 / 1 mud acid was pumped throug...
example 2
[0048]FIGS. 3 and 4 show a core flow experiment in which a split sandstone core was used and the affect of inert masking material was simulated. FIG. 3A shows the core with masking material in place, and FIG. 3B shows the core after etching. The 2.5 cm×15 cm inch core was cut in half along the core length; one half is shown as [12]. Teflon fibers [8] (about 0.08 cm×about 15 cm) were placed between the two pieces as shown in FIG. 3A. The pieces were then reassembled and loaded into a core holder, and a confining pressure of 13.8 MPa was applied. FIG. 4 shows the permeability when several fluids were injected into the gap between the two sandstone pieces in the core holder. A 5% ammonium fluoride solution was injected (triangles before about 7 min.) at a flow rate of 5 cc / min, then 12 / 6 mud acid at the same flow rate 9squares), and then 5% ammonium fluoride again at the same flow rate (triangles after about 17.5 min.). The permeability was clearly higher after the treatment of this si...
example 3
[0050]FIG. 5 shows a schematic of how a fracture would appear if created by the method of the invention. The fracture [4] in the formation [2] contains regions [6] that are not open to fluid flow. These regions are where the inert or reactive masking material is trapped when the fracture closes. The fracture face is protected from the formation dissolving agent at those locations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com