Mucosal vascular addressins and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
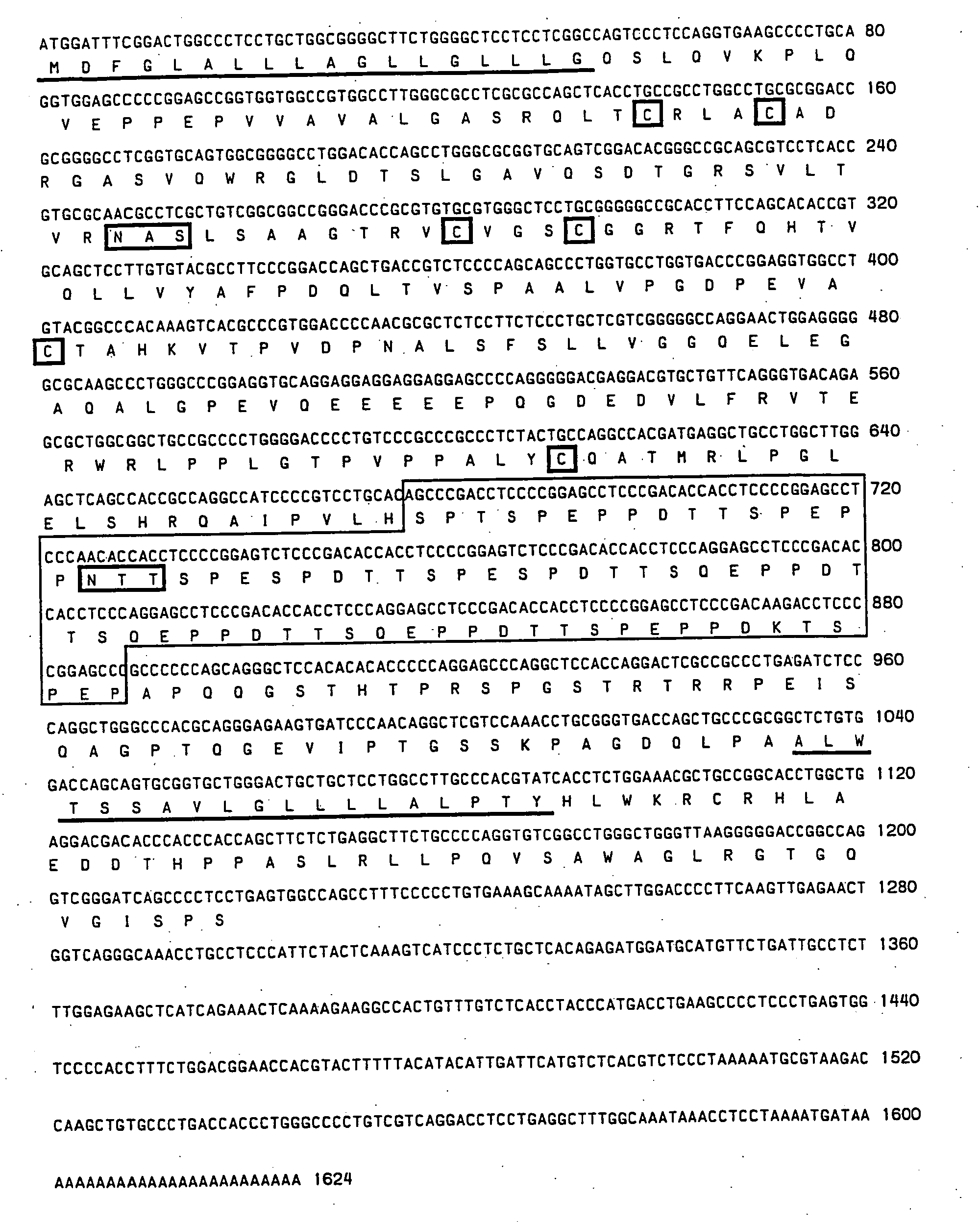
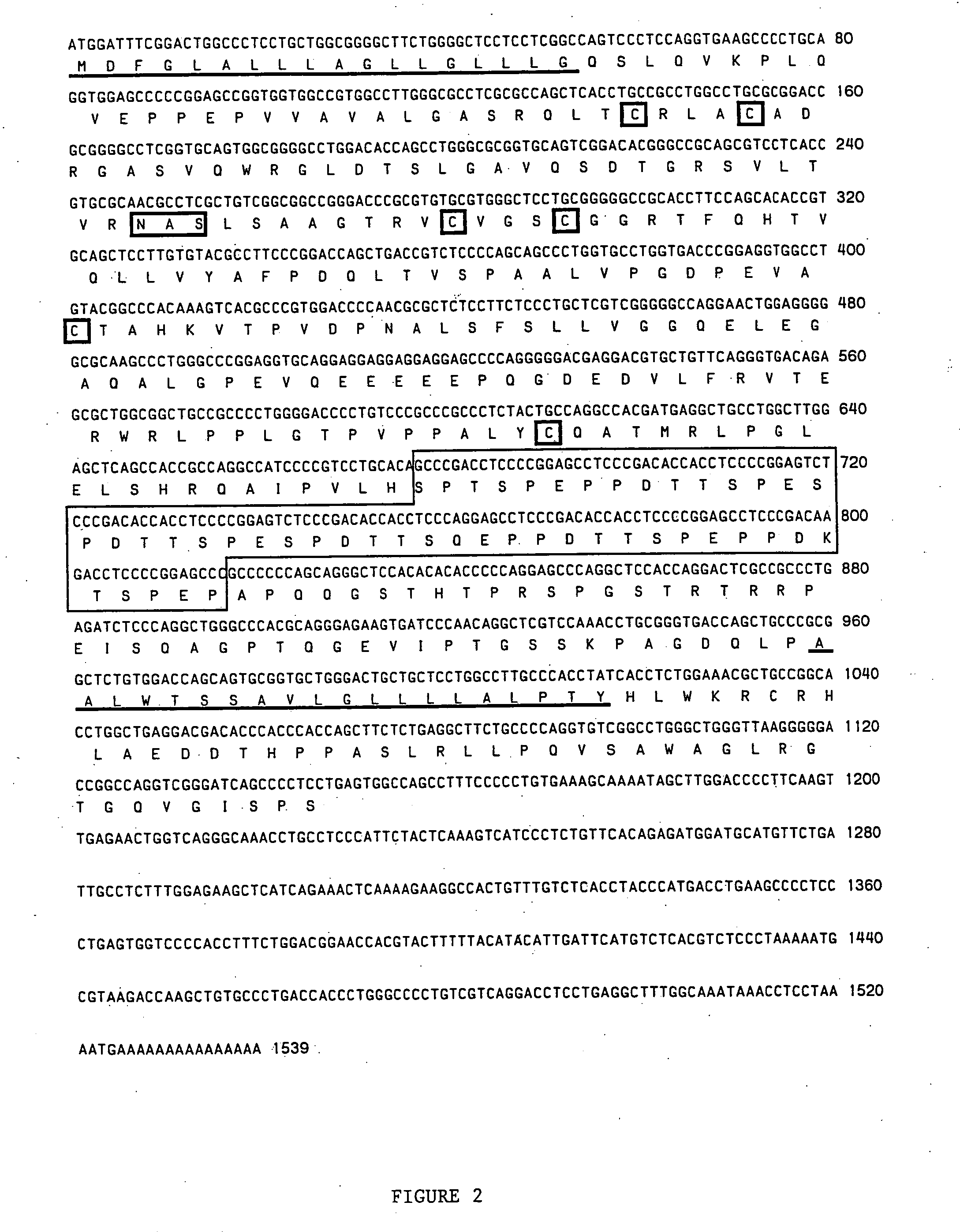
Cloning of Macaque and Human MAdCAM-1 cDNAs RNA Isolation and Selection of Message
[0125] Total RNA was isolated from (a) primate (macaque) mesenteric lymph nodes (MLN); (b) histologically normal human mesenteric lymph nodes; (c) human mesenteric lymph nodes (inflamed ileal nodes) from a patient with Crohn's disease; and (d) tissue culture cells by use of the CsTFA™ (cesium trifluoroacetate) reagent (Pharmacia; Cat. #17-087-02). Total RNA from mesenteric lymph node was obtained from two species of macaque (Macaca fascicularis, and Macaca mulatta), and was combined prior to isolation of poly-A RNA. Tissue was first snap frozen in liquid nitrogen and subjected to dounce homogenization in a solution consisting of 5.5 M guanidinium isothiocyanate, 25 mM sodium citrate, 0.5% sodium laurel sarcosine and 0.2 M 2-mercaptoethanol, while tissue culture cells (1-5×108) were washed once in phosphate buffered saline (PBS) and homogenized by pipetting. A clarified lysate was then layered on a cus...
example 2
Characterization of MAdCAM-1 Clones
[0169] Functional Adhesion Assays
[0170] Plasmids:
[0171] The following plasmids were used in the functional adhesion assays: (1) pSV-SPORT-1 (Gibco / BRL) or pcDNA-3 (Invitrogen) were used as controls; (2) murine MAdCAM-1 in pCDM8 (pCDMAD-7; Briskin, M. J., et al., Nature, 363:461 (1993)); (3) seven domain human VCAM-1 (Polte, T., et al., Nucleic Acids Res., 18:5901 (1990)) in pcDNA3 (pCD3VCAM); and (4) human MAdCAM-1 in pcDNA-3 (pCDhuMAd4) (see above).
[0172] Monoclonal Antibodies:
[0173] The following monoclonal antibodies (MAb) were used in the functional adhesion assays: (1) anti-murine MAdCAM-1 MAb MECA-367 (American Type Culture Collection (Rockville, Md.), Accession No. HB9478; Streeter, P. R., et al., Nature, 331:41 (1988); and U.S. Pat. No. 5,403,919 to Butcher); (2) anti-human VCAM-1 MAb 2G7 (American Type Culture Collection (Rockville, Md.); Graber, N. T., et al., J. Immunol., 145:819-830 (1990)); (3) anti-murine α4β7 MAb DATK 32 (Andrew...
example 3
Design and Functional Analysis of a Human MAdCAM-1-IgG Chimera Construction of MAdCAM-IgG Chimera
[0198] Human MAdCAM-1 clone 4 cDNA in pCDNA3 (Invitrogen, San Diego, Calif.), called pcD3huMAd4 (Example 1) was used as a template for PCR amplification of extracellular regions of human MAdCAM-1 to be fused with the constant region of human IgG1. Primer HUMADIG4 / 2 (SEQ ID NO:11), which contains the 5′ end of human MAdCAM-1 coding sequence (ATG codon, bold), was synthesized:
HindIII5′-GGAAGCTTCCACCATGGATTTCGGACTGGCCC-3′
[0199] This 5′ primer was used in conjunction with a 3′ primer designated HUMADIG2 (SEQ ID NO:12) to amplify regions encoding the two amino-terminal immunoglobulin-like (Ig) domains of human MAdCAM-1. Primer HUMADIG2 (SEQ ID NO:12), which contains a portion complementary to coding strand nucleotides 667-683 of SEQ ID NO:1, has the following sequence:
SpeI5′-CCGACTAGTGTCGGGCTGTGCAGGAC-3′
[0200] Alternatively, the 5′ primer was used in conjunction with 3′ primer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com