Formation of barrier layer
a barrier layer and layer technology, applied in the field of barrier layers, can solve the problems of structural weakness, difficult handling of adhesion barrier products, and difficult application to the targeted location of adhesion prevention and adhesion barrier products,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
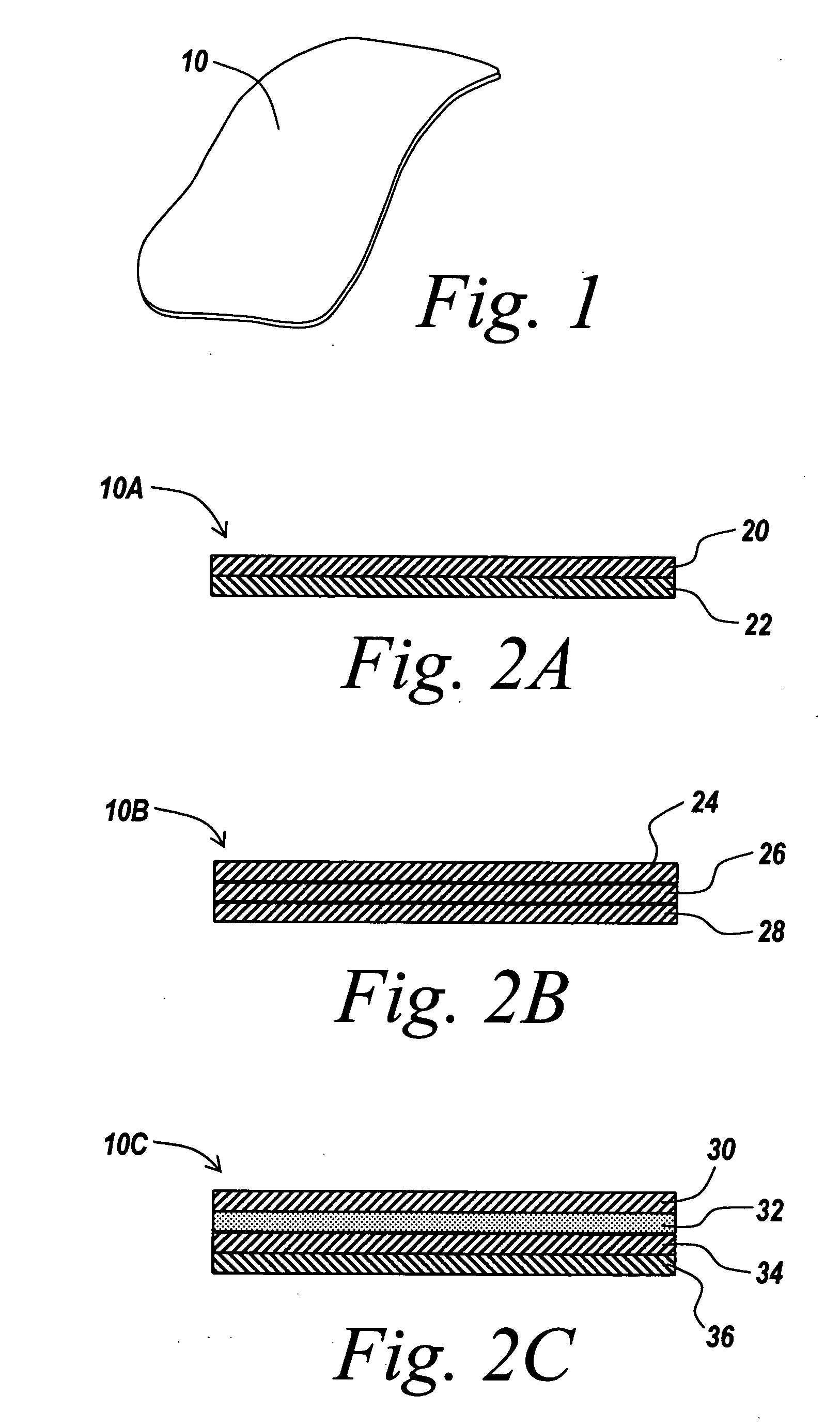
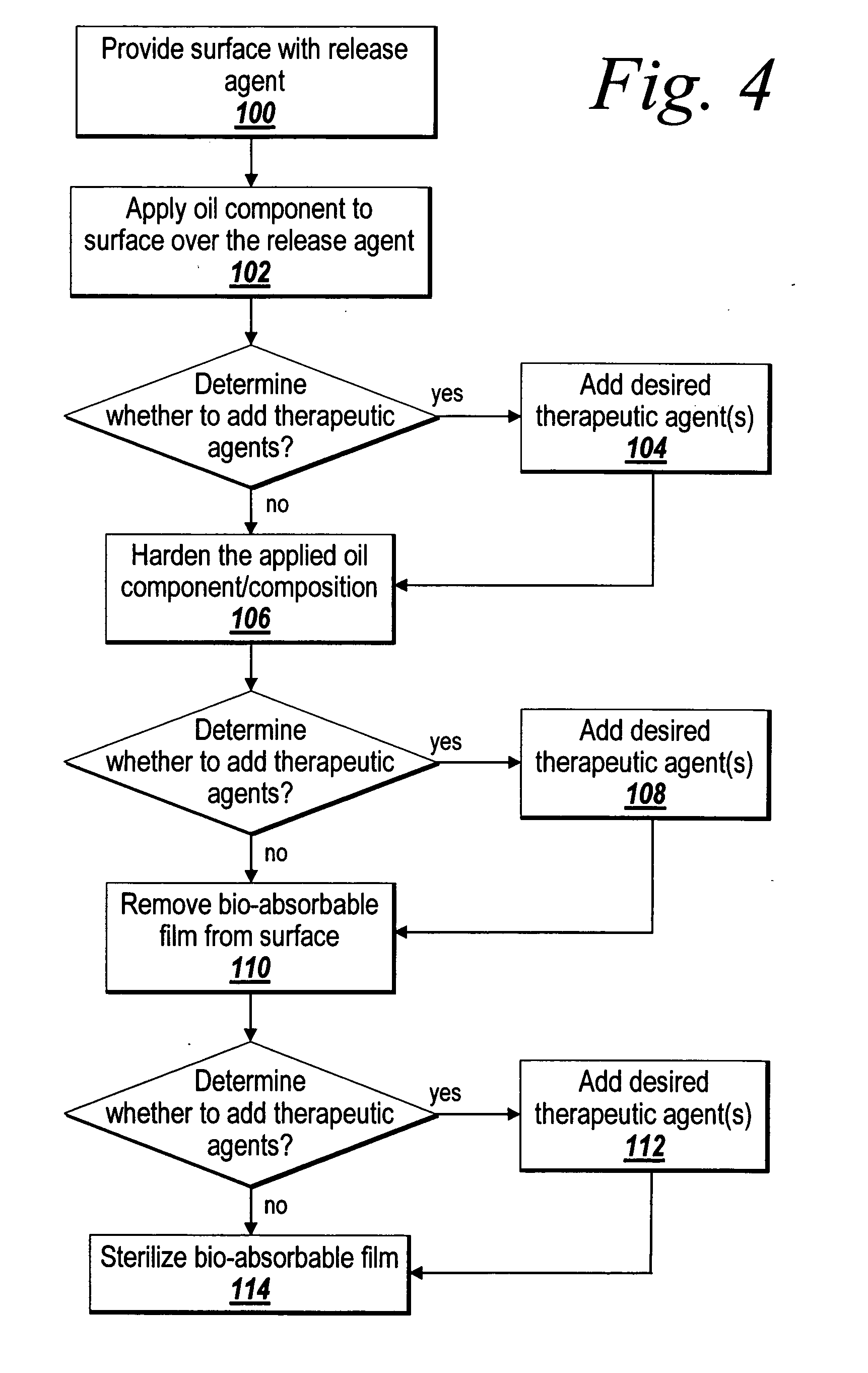
[0036] The present invention relates to the provision of a barrier layer that can exhibit anti-inflammatory properties, non-inflammatory properties, and anti-adhesion properties, and corresponding method of making. The barrier layer can be its own medical device (i.e., a stand alone film), or the barrier layer can be combined with another medical device to provide anti-adhesion characteristics, in addition to improved healing and delivery of therapeutic agents. The barrier layer is generally formed of a naturally occurring oil, or an oil composition formed in part of a naturally occurring oil. In addition, the oil composition can include a therapeutic agent component, such as a drug or other bioactive agent. The barrier layer is implantable in a patient for short term or long term applications, and can include controlled release of the therapeutic agent. As implemented herein, the barrier layer is a non-polymeric cross-linked gel derived at least in part from a fatty acid compound. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| biocompatible | aaaaa | aaaaa |
| bio-absorbable | aaaaa | aaaaa |
| anti-adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com