Nitrogen-containing cyclic compound and pharmaceutical composition containing the compound
a cyclic compound and cyclic compound technology, applied in the field of new compounds, can solve the problems of insufficient therapeutic effects of the drugs used, inapplicability of argatroban to lacuna infarction, and inability to achieve the effects of preventing, treating or improving neural disease or pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
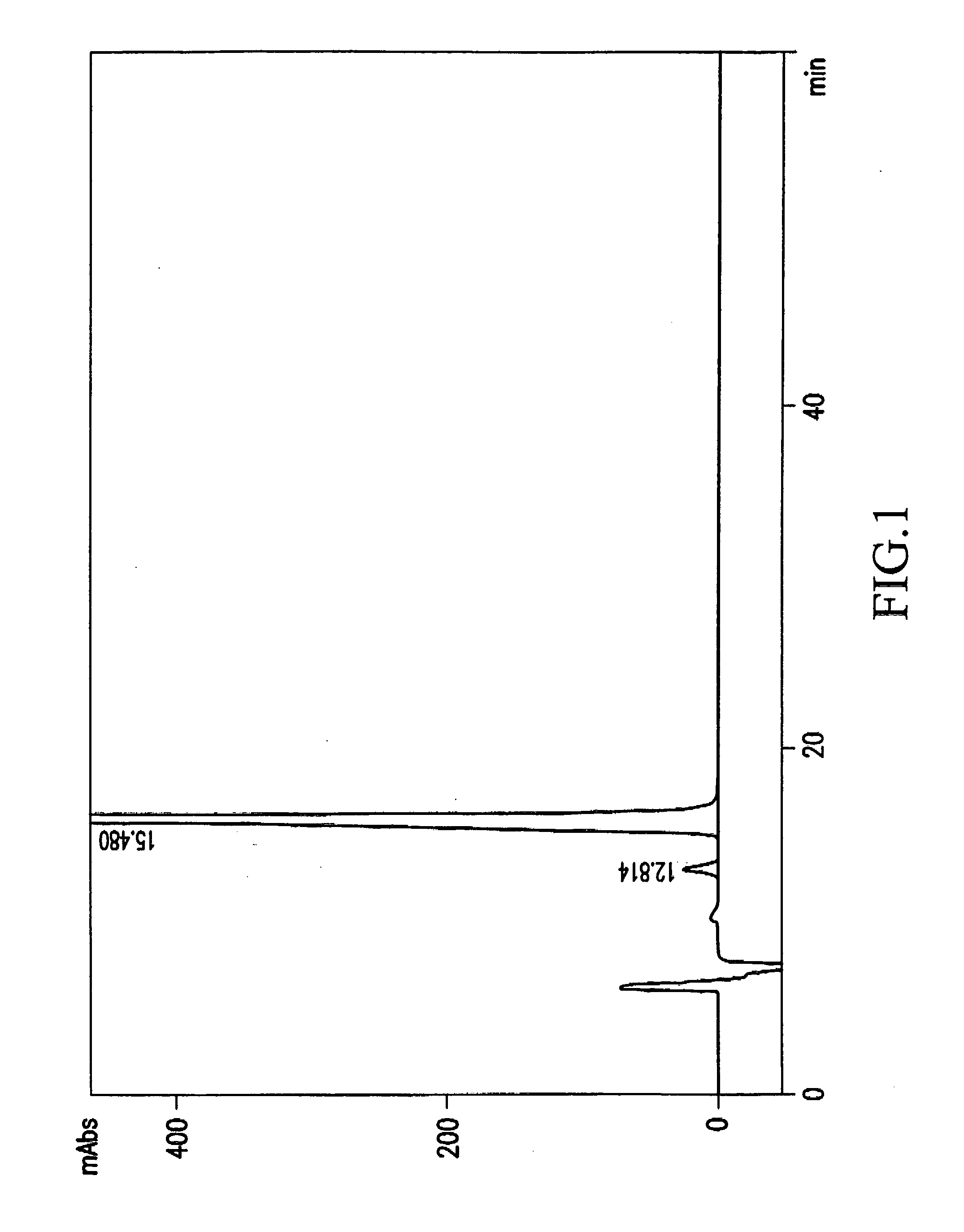
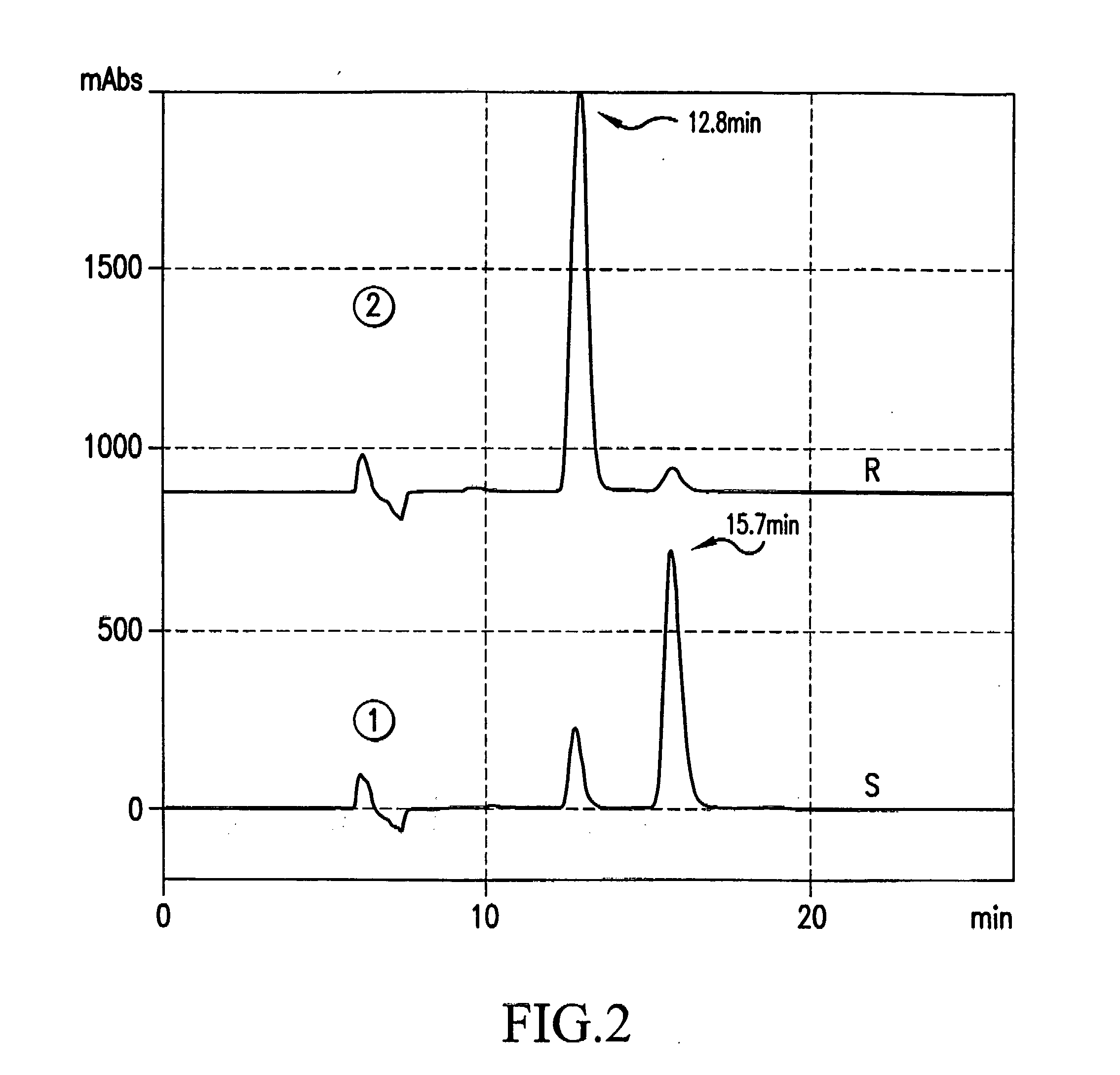
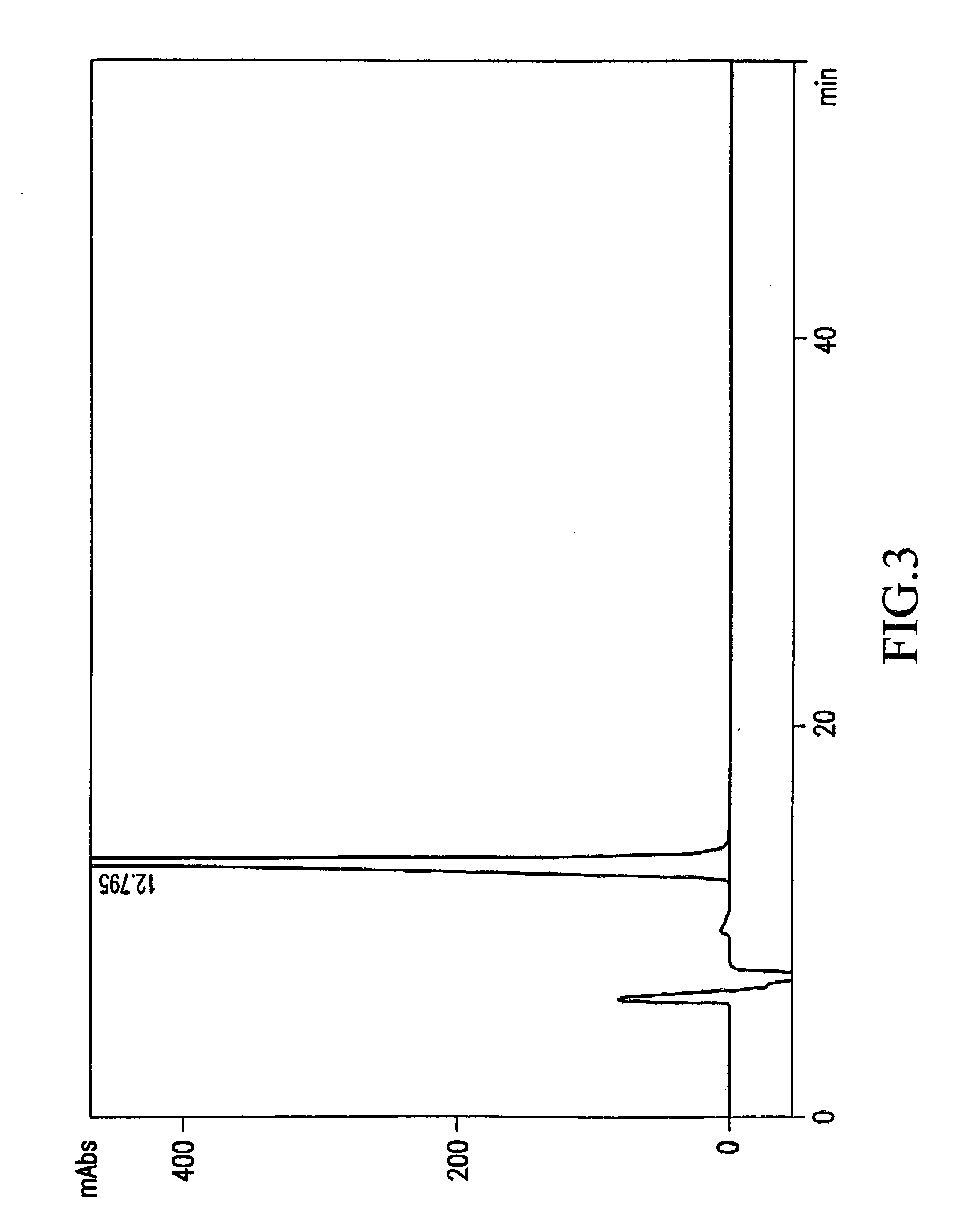
Image
Examples
reference example 1
2-[(4-Cyano-5-methyl-4-phenyl)hexyl]-5-benzyl-2,5-diazabicyclo[2,2,1]heptane
[0165]
[0166] The title compound was obtained as a pale brown oil in accordance with the method described in Example 15 (15%).
[0167]1H-NMR (400 MHz, CDCl3) δ 0.78 (d, J=6.8 Hz, 3H), 1.04-1.16 (m, 1H), 1.20 (d, J=6.8 Hz, 3H), 1.45-1.57 (m, 1H), 1.64 (dd, J=9.6 Hz, J=33.6 Hz, 2H), 1.94 (dt, J=4.4 Hz, J=12.4 Hz, 1H), 2.07-2.23 (m, 2H), 2.30-2.38 (m, 1H), 2.50-2.71 (m, 5H), 3.19 (d, J=14 Hz, 2H), 3.66 (q, J=14 Hz, 2H), 7.19-7.40 (m, 10H).
reference example 2
3-Methyl-2-(2-naphthyl)butyronitrile
[0168]
[0169] 3.00 g (17.9mmol) of 2-naphthylacetonitrile was dissolved in 10 ml of dimethyl sulfoxide, and 2.43 g (19.7 mmol) of 2-bromopropane, 330mg (0.90 mmol, cat) of tetra-n-butylammonium iodide and 10 ml of 50% potassium hydroxide were successively added thereto. After completion of the reaction, brine was added, and the mixture was extracted with ether. The organic layer was washed with brine, dried over magnesium sulfate and evaporated, to give a crude product. The crude product was subjected to 150 g of silica gel (ethyl acetate:hexane=1:10), to give 2.42 g (11.6 mmol, 64.6%) of the title compound as a yellow oil.
[0170]1H-NMR (400 MHz, CDCl3) δ 1.07 (d, J=6.8 Hz, 3H), 1.11 (d, J=6.8 Hz, 3H), 2.10-2.30 (m, 1H), 3.84 (d, J=3.84 Hz, 1H), 7.38 (dd, J=1.8 Hz, 8.6 Hz, 1H), 7.48-7.55 (m, 2H), 7.79-7.88 (m, 4H)
reference example 3
4-Cyano-5-methyl-4- (2-naphthyl)hexanol
[0171]
[0172] 1.00 g (4.78 mmol) of 3-methyl-2-(2-naphthyl)butyronitrile was dissolved in 20 ml of dimethylformamide, 191 mg (4.78 mmol, 60% by weight) of sodium hydride was added thereto, and the mixture was heated. After 30 minutes, it was cooled to a room temperature, 0.93 ml (4.00 mmol) of (3-bromopropoxy)-tert-butyldimethylsilane was added thereto. After completion of the reaction, brine was added thereto, and the mixture was extracted with ethyl acetate. The organic layer was washed with brine, dried over magnesium sulfate and evaporated, to give a crude product. The crude product was subjected to 50 g of silica gel (ethyl acetate:hexane=1:18), to give 1.40 g of a mixture of the objective product, a raw material and an impurity. The mixture was used for the following reaction without purification. Namely, 1.40 g of the abve-mentioned crude 4-cyano-5-methyl-5-(2-naphthyl)hexanoxy-tert-butyldimethylsilane was dissolved in 20 ml of tetrahydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com