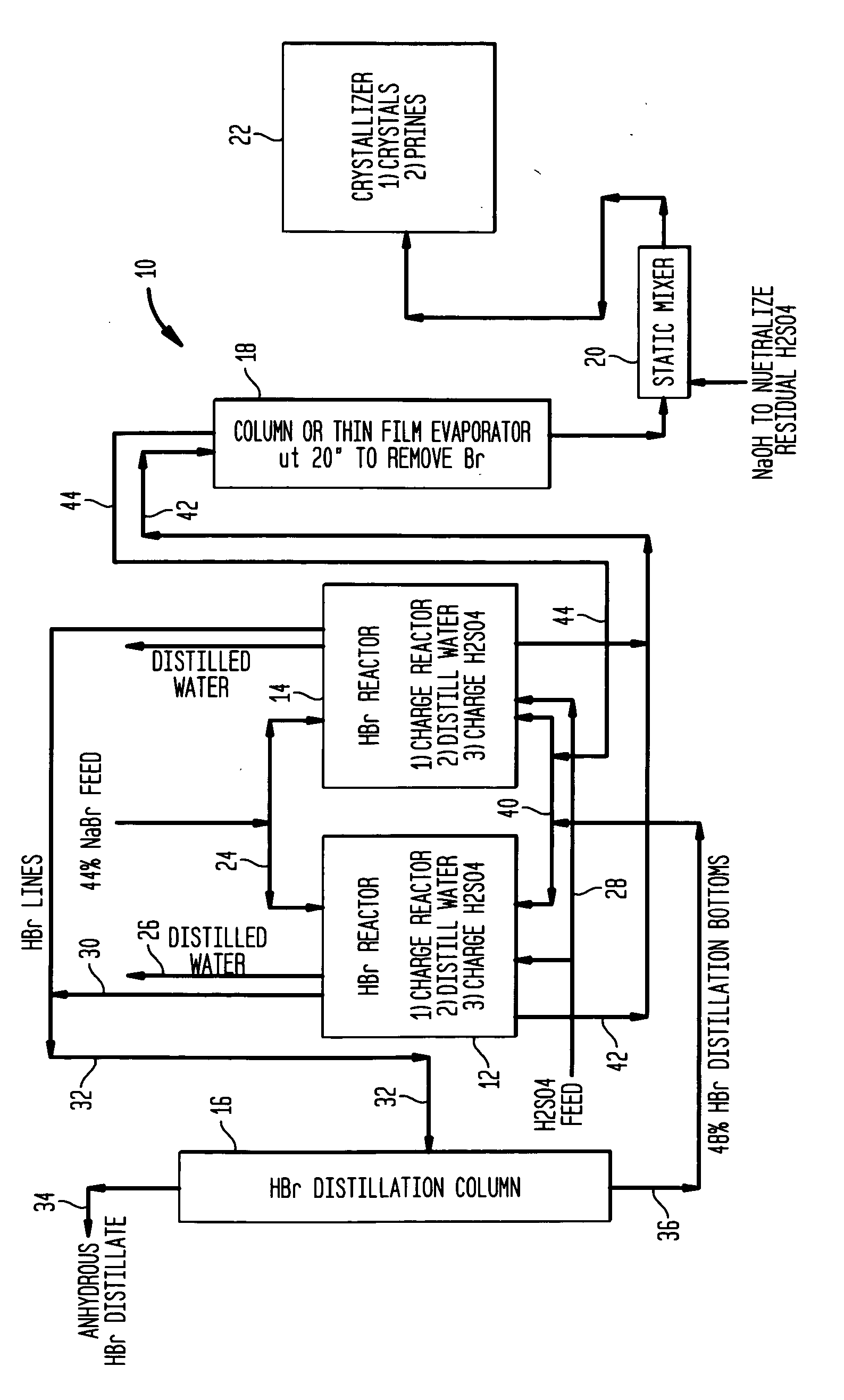
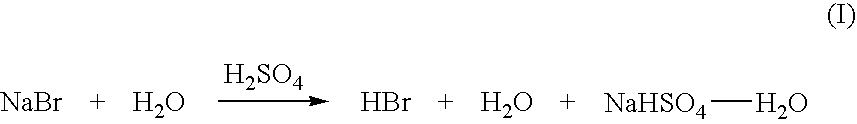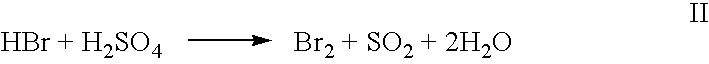High yield co-production of anhydrous hydrogen bromide and sodium bisulfate
a technology of anhydrous hydrogen bromide and sodium bisulfate, which is applied in the direction of lithium compounds, sulfur compounds, alkali metal sulfites/sulfates, etc., can solve the problems of high boiling water/hbr azeotrope stream, hydrogen bromide, and limited commercial application of azeotropic solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Aqueous slurry with NaBr and water: A slurry of 258 gm sodium bromide and 58 gm water was prepared and added to a 500 ml round bottom flask. To this was added 238 gm of concentrated sulfuric acid over a period of 1.5 hours at a temperature of approximately 120° C. Upon addition of 15% of the sulfuric acid hydrogen bromide gas began to off gas and was collected in a water trap. The reaction bottoms were then heated 145° C. once all the sulfuric acid was added to drive off additional HBr.
[0040] The reaction effluents were as follows: [0041] 205 gm of HBr was collected and consisted of 90% HBr and 10% water. [0042] 334.3 gm of sodium bisulfate was collected and contained 4.0% Br-
example 2
[0043] An aqueous slurry of NaBr in 48% HBr: A slurry of 600 gm sodium bromide and 110 gm of 48% aqueous hydrogen bromide was prepared and added to a 1000 ml round bottom flask. To this was added 637 gm of concentrated sulfuric acid over a period of 1.5 hours. The initial temperature at the beginning of the acid addition was 70° C. and was ramped up to 140° C. at the end of the acid addition. Hydrogen bromide was generated immediately upon the addition of sulfuric acid and was collected in a water trap.
[0044] The reaction effluents were as follows: [0045] 526 gm of HBr was collected and consisted of 90% HBr and 10% water. [0046] 820 gm of sodium bisulfate was collected and contained 3.9% Br-.
example 3
[0047] An aqueous mixture ofNaBr and NaHS04-H2O: A slurry of 600 gm sodium bromide with 62 gm of 48% aqueous hydrogen bromide 59 gm NaHSO4 and 8 gm water was prepared and added to a 1000 ml round bottom flask. To this was added 638 gm of concentrated sulfuric acid over a period of 45 minutes. The temperature was held at 120° C. Hydrogen bromide was generated almost immediately upon the addition of sulfuric acid and was collected in a water trap.
[0048] The reaction effluents were as follows: [0049] 487 gm of HBr was collected and consisted of 98% HBr and 2% water. [0050] 887 gm of sodium bisulfate was collected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight % | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com