Grinding method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0041] In this experiment, the effect of various starvation (very low) doses of sodium hexametaphosphate dispersant on the slurry properties of ground calcium carbonate was measured.
[0042] Ground calcium carbonate was prepared by laboratory slurry sandgrinding at 250 kWh / t at low solids (25 weight-%) from a coarse calcium carbonate feed. Three batches of sandground material were prepared containing, respectively, dispersant at 0.1%, 0.2% and 0.3% by weight of dry calcium carbonate. The three batches were then dewatered by low pressure piston pressing at 250 psi, to obtain a cake. The permeability and resistance of the cake was measured during pressing in each case, using the test methods described below. Each of the three cakes was then divided into two samples and the cake samples suspended (slurry makedown) using 0.5% and 0.6% partially neutralised sodium polyacrylate dispersant solution (60% neutralisation), to obtain high solids slurries at 72-73 weight-% solids. Brookfield 100...
example 2
[0049] In this experiment, the effect of various starvation (very low) pre-grind doses of the dispersants sodium hexametaphosphate (NaHex), sodium polyacrylate DP2695 (available from Ciba Chemicals) and combinations thereof on the slurry properties of ground calcium carbonate was measured.
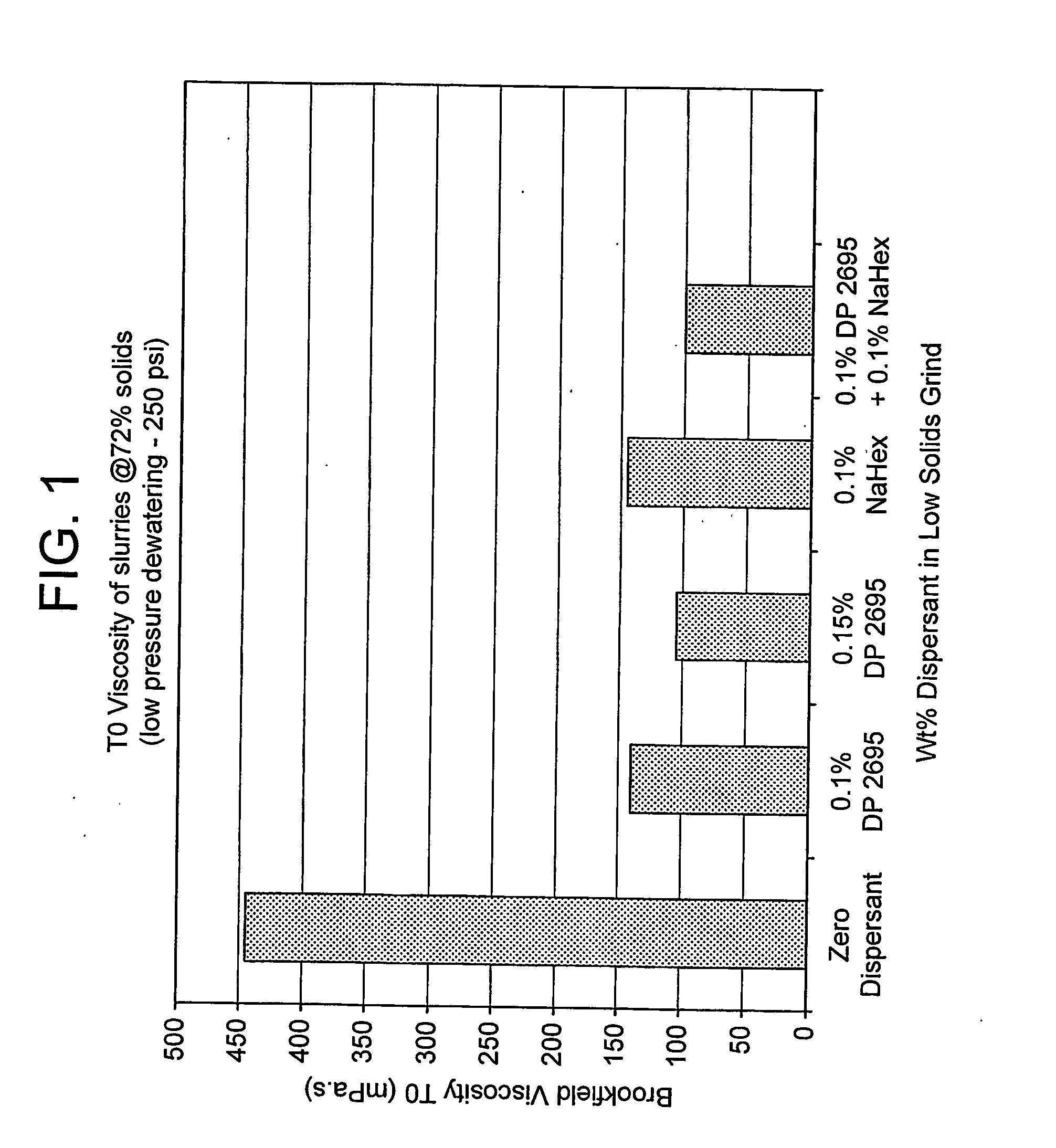
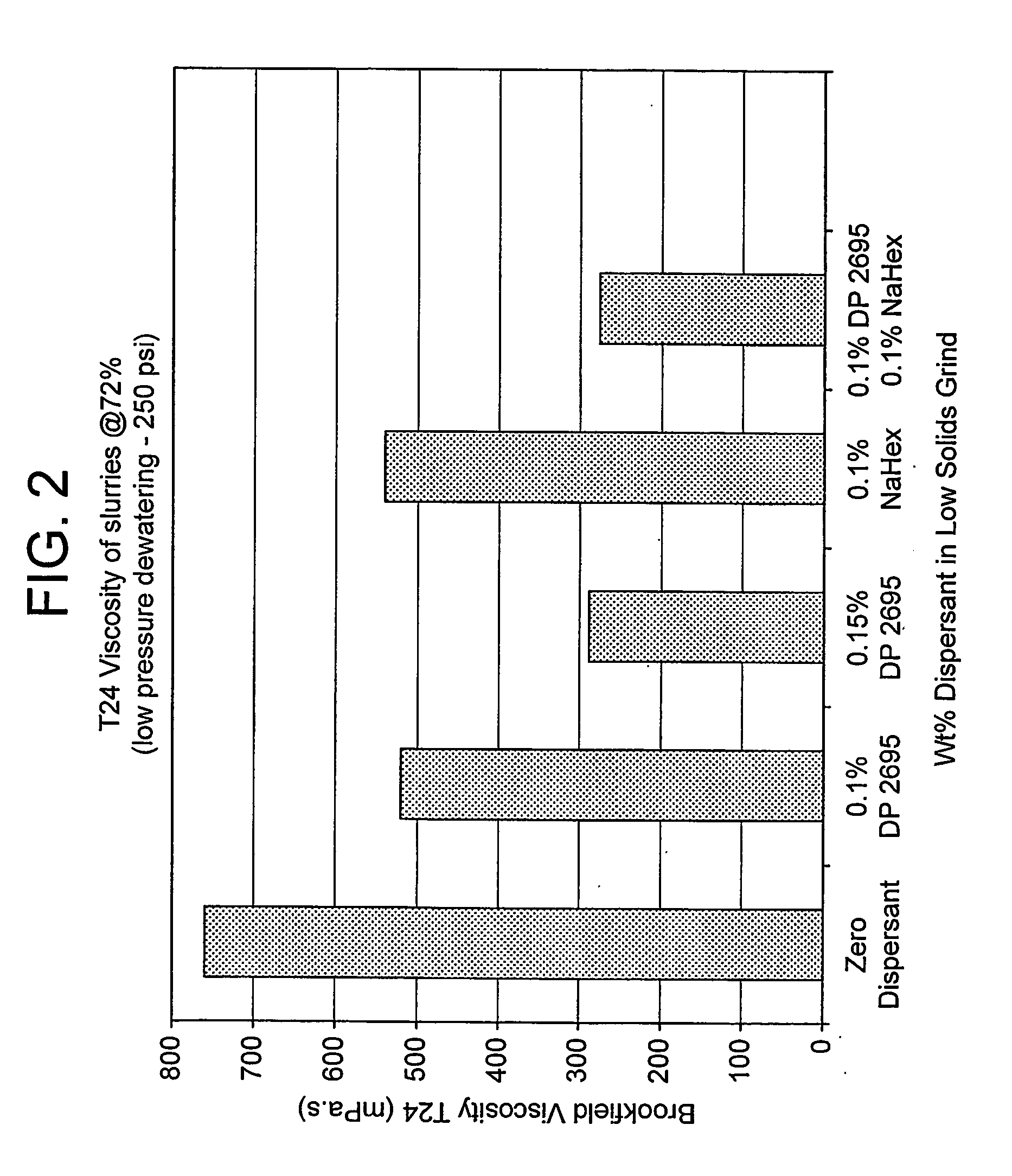
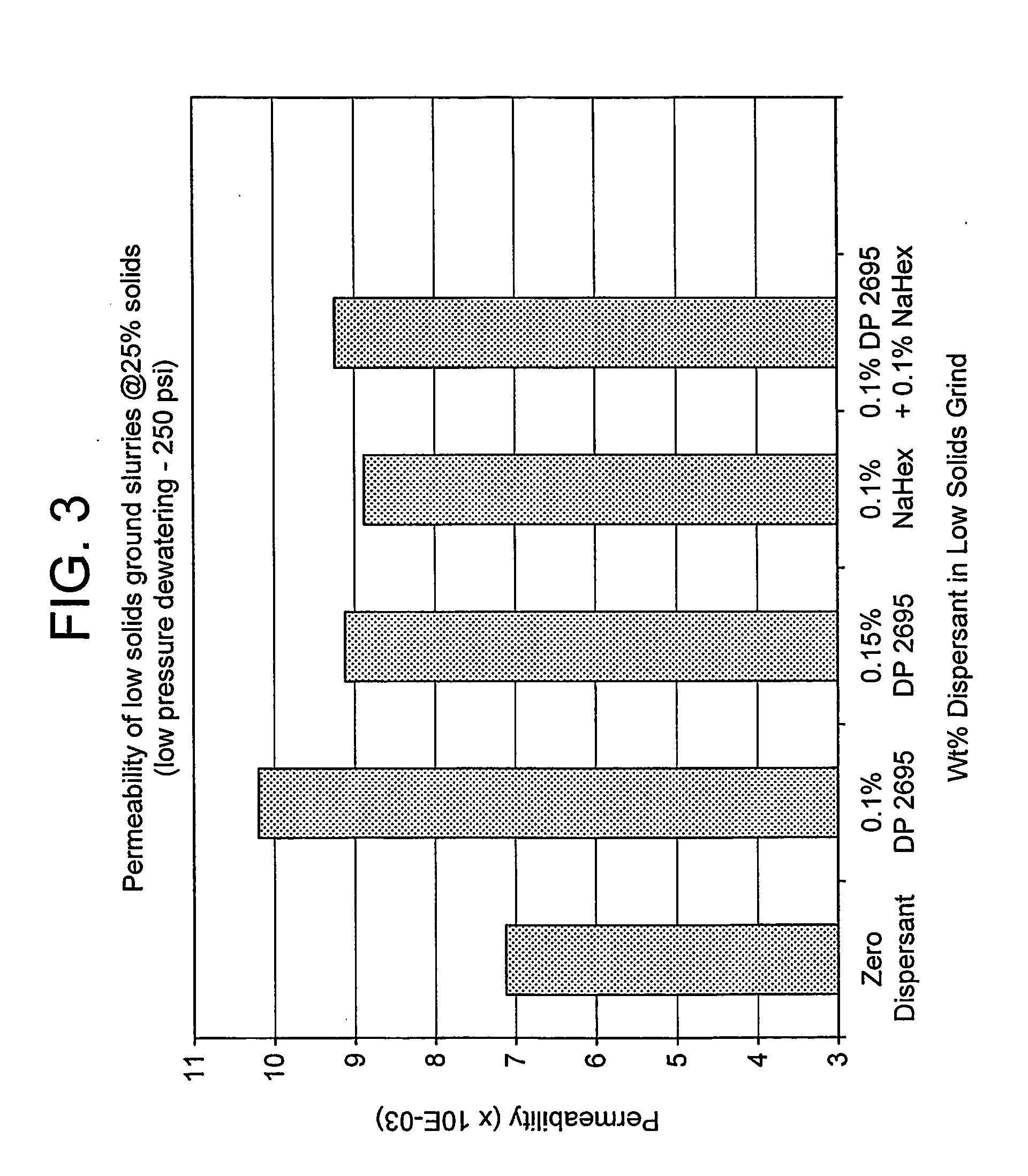
[0050] The method was essentially that described above for Example 1, but using different dispersants, as shown in FIGS. 1 to 3 of the drawings, and with a total dose of added dispersant equal to 0.6%.
Discussion and Conclusions
[0051] The results of the T=0 and T=24 hours Brookfield 100 rpm viscosity measurements on the 72% solids slurry obtained after makedown are illustrated in FIGS. 1 and 2. The results of the cake permeability measurements on low pressure piston pressing at 25 weight-% solids are shown in FIG. 3.
[0052] The results in FIG. 1 show that a pre-grind dose of 0.1% and 0.15% sodium polyacrylate gave good T=0 viscosity. However, all the slurries treated with a starvation dose of di...
example 3
[0055] In this Example, the effect of adding sodium hexametaphosphate on the brightness of ground calcium carbonate slurries was measured.
Experimental
[0056] Samples of two ground calcium carbonates A and B were used, having respective psd as follows: A—99% by weight less than 2 μm, 90% by weight less than 1 μm, 70% by weight less than 0.5 μm, 35% by weight less than 0.25μm; B—95% by weight less than 2 μm, 75% by weight less than 1 μm, 40% by weight less than 0.5 μm, 15% by weight less than 0.25 μm. Each carbonate sample was split into two portions and each portion was diluted to provide about 30 and 20 wt % solids suspensions (slurries) of carbonates A and B. To samples of each suspension, 200 ppm of powdered iron was added and to selected ones of those samples 0.1% sodium hexametaphosphate was added (see Table 2) Oxygen was applied to selected slurry samples for 30 minutes at about 10 l / min per sample at temperatures of 22° C. and 50° C. Following treatment, the samples were flo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com