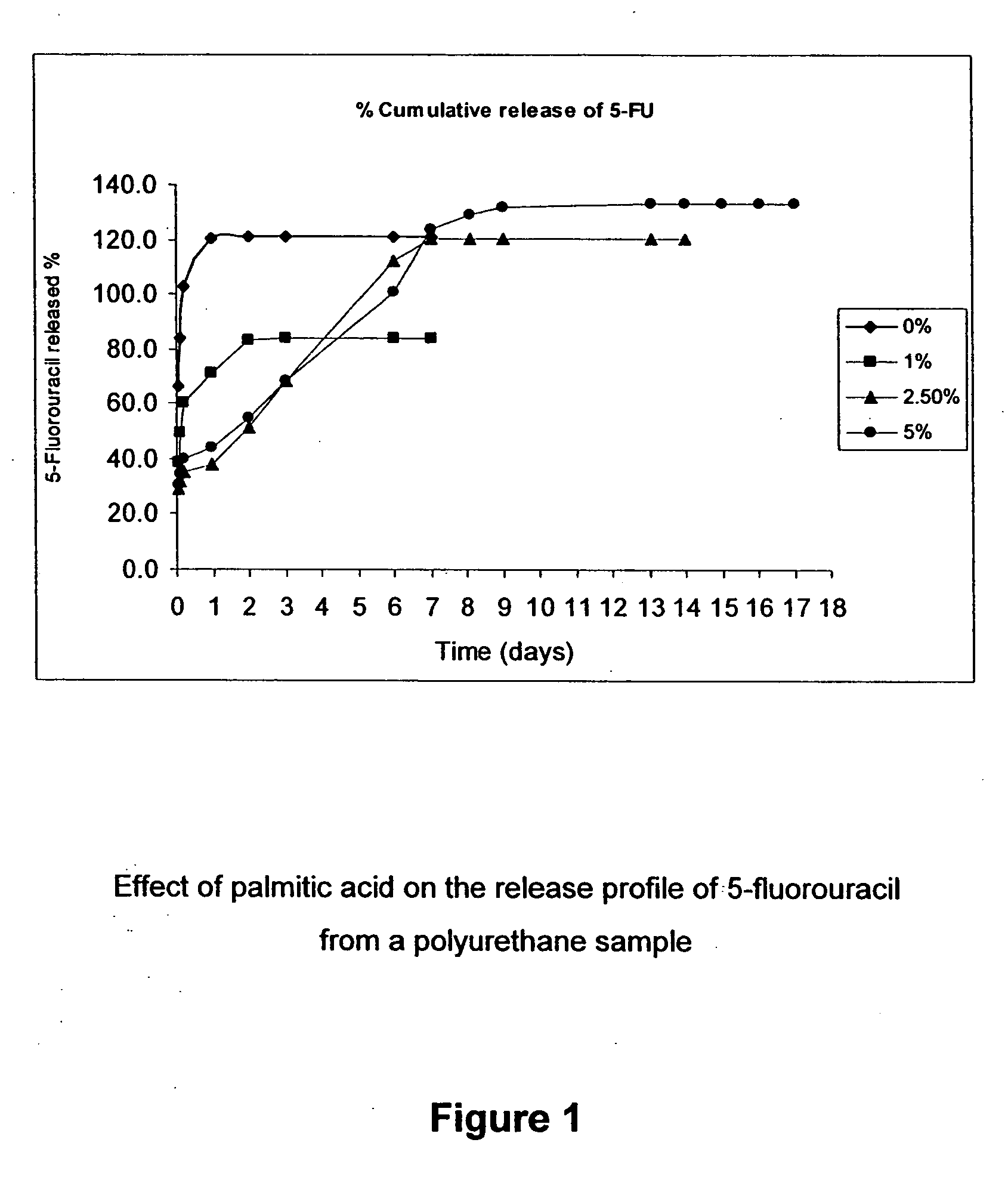
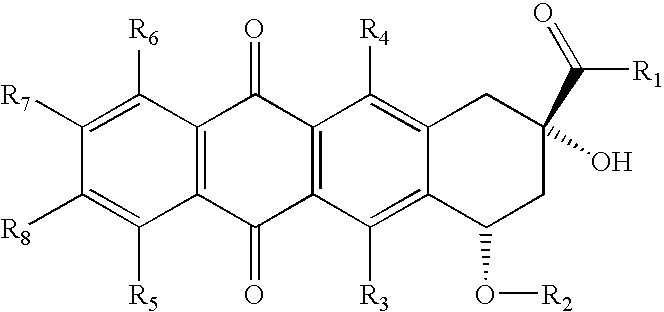
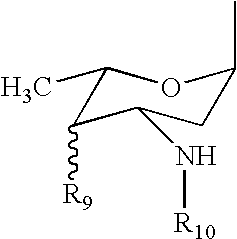
Compositions and methods for coating medical implants
a technology of medical implants and compositions, applied in the direction of prosthesis, catheters, therapy, etc., can solve the problems of increasing morbidity for patients, over $2 billion annually, and major healthcare problems affecting the infection of medical implants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MIC Determination by Microtitre Broth Dilution Method
[0335] A. MIC Assay of Various Gram Negative and Positive Bacteria
[0336] MIC assays were conducted essentially as described by Amsterdam, D. 1996. Susceptibility testing of antimicrobials in liquid media, p. 52-111. In Loman, V., ed. Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, Md. Briefly, a variety of compounds were tested for antibacterial activity against isolates of P. aeruginosa, K. pneumoniae, E. coli, S. epidermidus and S. aureus in the MIC (minimum inhibitory concentration assay under aerobic conditions using 96 well polystyrene microtitre plates (Falcon 1177), and Mueller Hinton broth at 37° C. incubated for 24 h. (MHB was used for most testing except C721 (S. pyogenes), which used Todd Hewitt broth, and Haemophilus influenzae, which used Haemophilus test medium (HTM)) Tests were conducted in triplicate. The results are provided below in Table 1.
TABLE 1Minimum Inhibitory Concentrations...
example 2
Catheter—DIP Coating—Non-Degradable Polymer
[0339] A coating solution is prepared by dissolving 20 g ChronoFlex Al 85A (CT Biomaterials) in 100 mL DMAC:THF (40:60) at 50° C. with stirring. Once dissolved, the polymer solution is cooled to room temperature. 20 mg mitoxantrone is added to 2 mL of the polyurethane solution. The solution is stirred until a homogenious mixture is obtained. Polyurethane 7 French tubing is dipped into the polymer / drug solution and then withdrawn. The coated tube is air dried (80° C.). The sample is then dried under vacuum to further reduce the residual solvent in the coating.
example 3
Catheter—Dip Coating—Degradable Polymer
[0340] A coating solution is prepared by dissolving 2 g PLG (50:50) in 10 mL dichloromethane:methanol (70:30). Once dissolved, 20 mg mitoxantrone is added to the polymer solution. Once the solution is a homogeneous solution, polyurethane 7 French tubing is dipped into the solution and then withdrawn. The coated tube is air dried. The sample is then dried under vacuum to further reduce the residual solvent in the coating.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com