Randomized libraries of zinc finger proteins
a technology of zinc finger protein and library, applied in the field of libraries of randomized zinc finger proteins, can solve the problems of limited phenotype, cumbersome method development of high throughput screening assay using these methods, and inability to use assays for transdominant genes, etc., to achieve reliable, efficient means for regulating gene expression, and regulate gene expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protocol for Preparation and Screening Using a Randomized Zinc Finger Protein Library Generated by Finger Grating
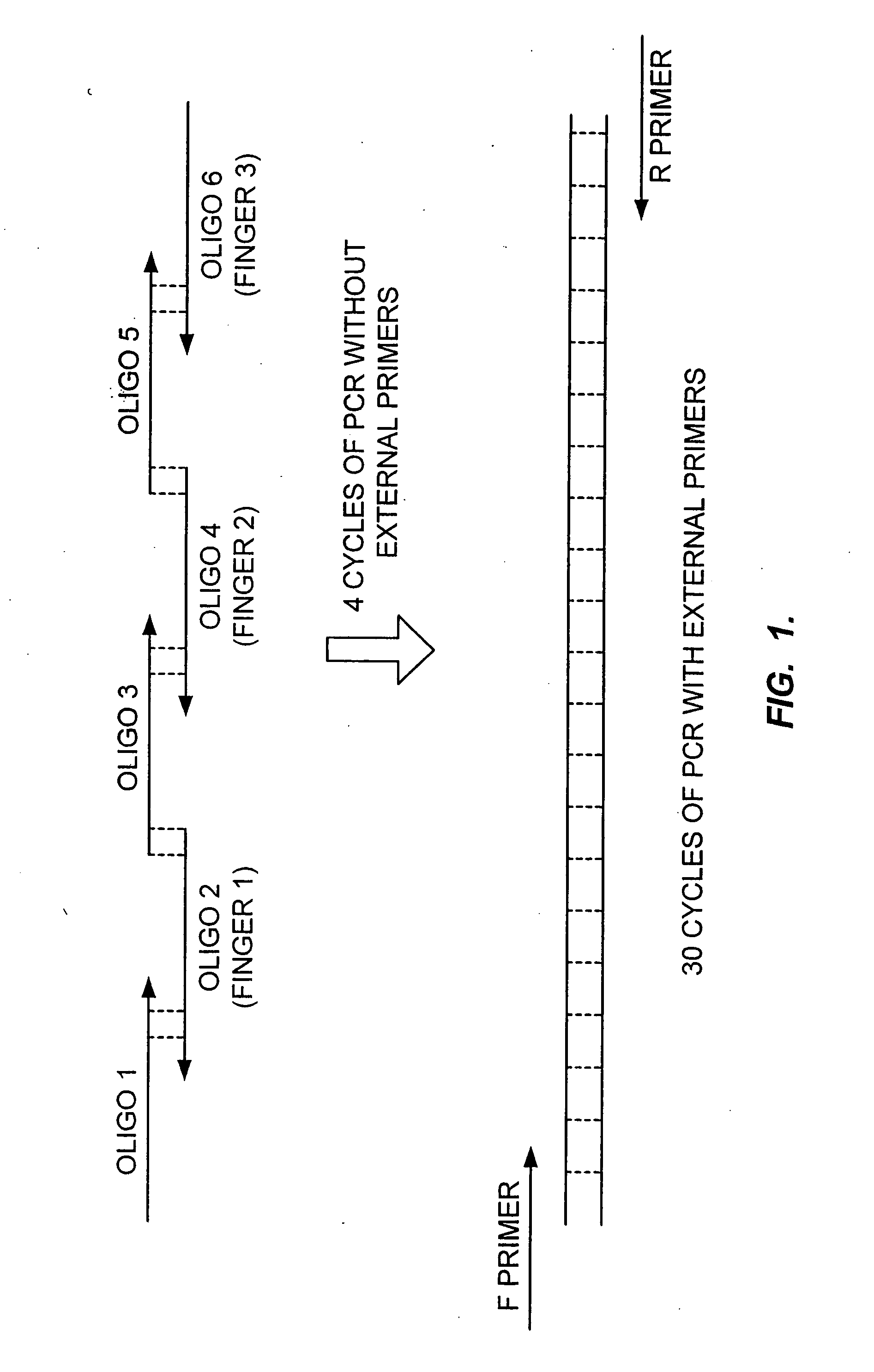
[0134] A. Generation of a Library Using Finger Grafting
[0135] A library of 12 different helices compatible with 5 different finger positions will be created and assembled into zinc finger proteins using a method similar to that currently used to assemble engineered 3 finger proteins (see, e.g., U.S. Ser. No. 09 / 229,037 filed Jan. 12, 1999, and U.S. Ser. No. 09 / 229,007, filed Jan. 12, 1999). Randomness will be confirmed by sequencing a representative sample. Of this library, 250,000 individual bacterial transformants will be picked and archived. The individual transformants will be combined into pools of 8 and cloned into a viral delivery vector (such as an adenoviral vector).
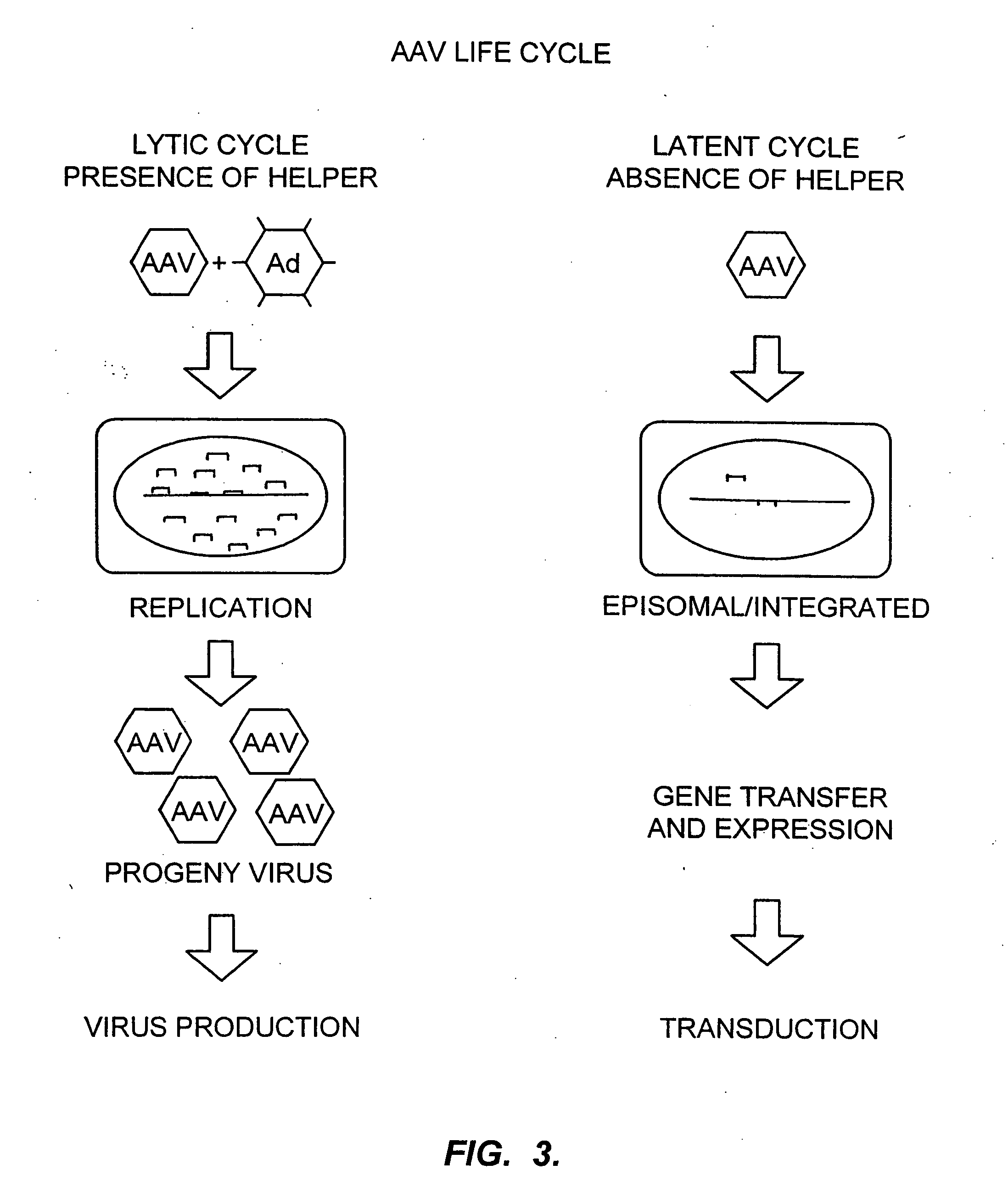
[0136] Viral delivery particles will be produced from each pool (there are 31,250 different pools) and tested in an appropriate assay for the identification of a desired phenotype. Assays could be ...
example 2
Protocol for Preparation and Screening Using a Randomized Zinc Finger Protein Library Generated by Codon Doping
[0154] A. Preparation of the Library Using Codon Doping
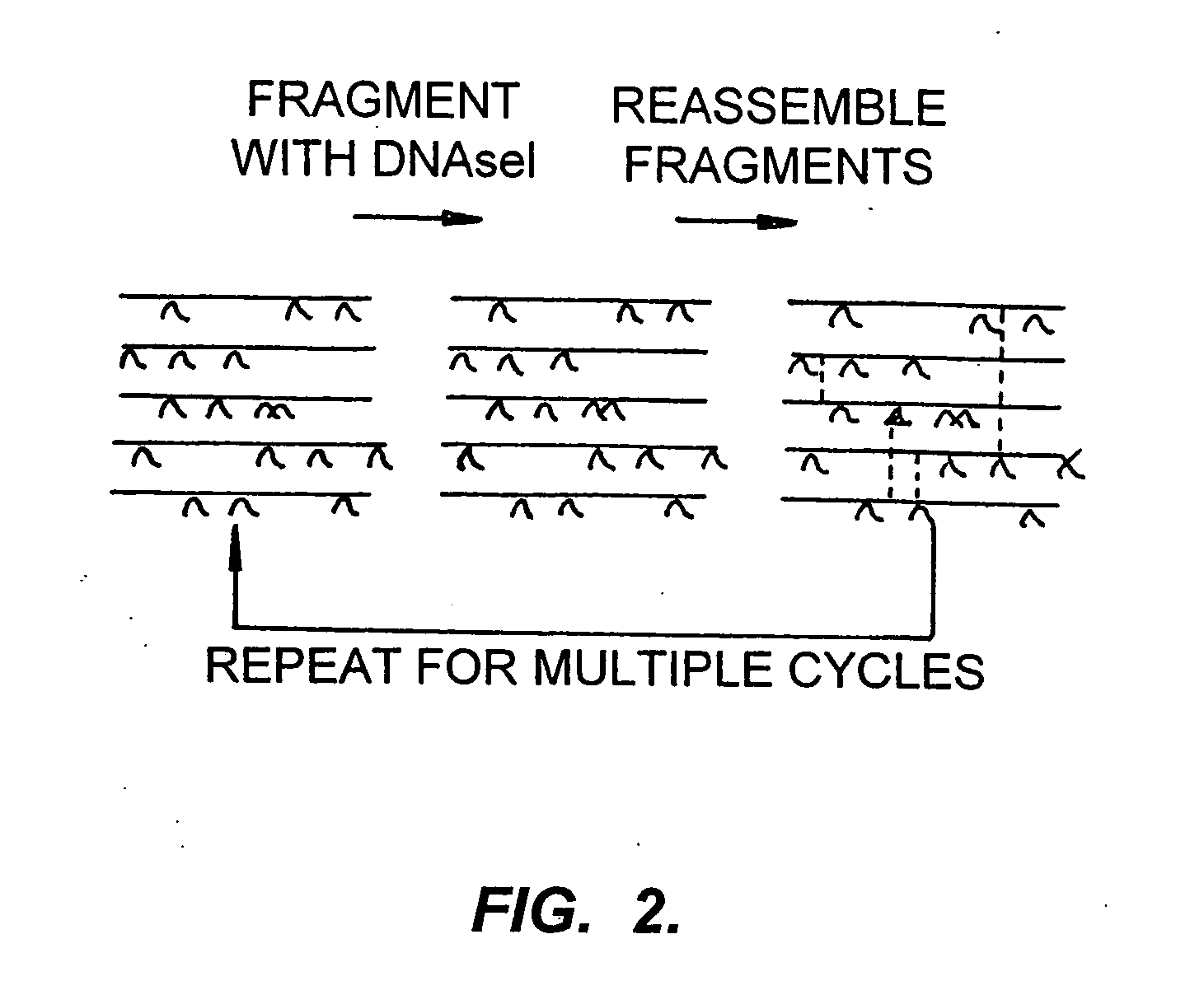
[0155] As described above, each zinc finger binds three nucleotides using four critical amino acids in the recognition helix. If each base in the codons for these amino acids was simply randomized, it would generate a library of 412 clones (1.7 million). This number is already in excess of a desired library limit of about one million to about 10 or 100 million clones and only concerns one finger (and three are to be used in these methods). However, it is not necessary to use completely random codons. Because of the redundancy of the genetic code, schemes of semi-randomization can generate representatives of all, or nearly all codons. This strategy is thus called a codon doping scheme.
[0156] One randomization scheme uses VNS instead of NNN, where N=any base, V=A or G and S=G or C. All of the codons are represented by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com