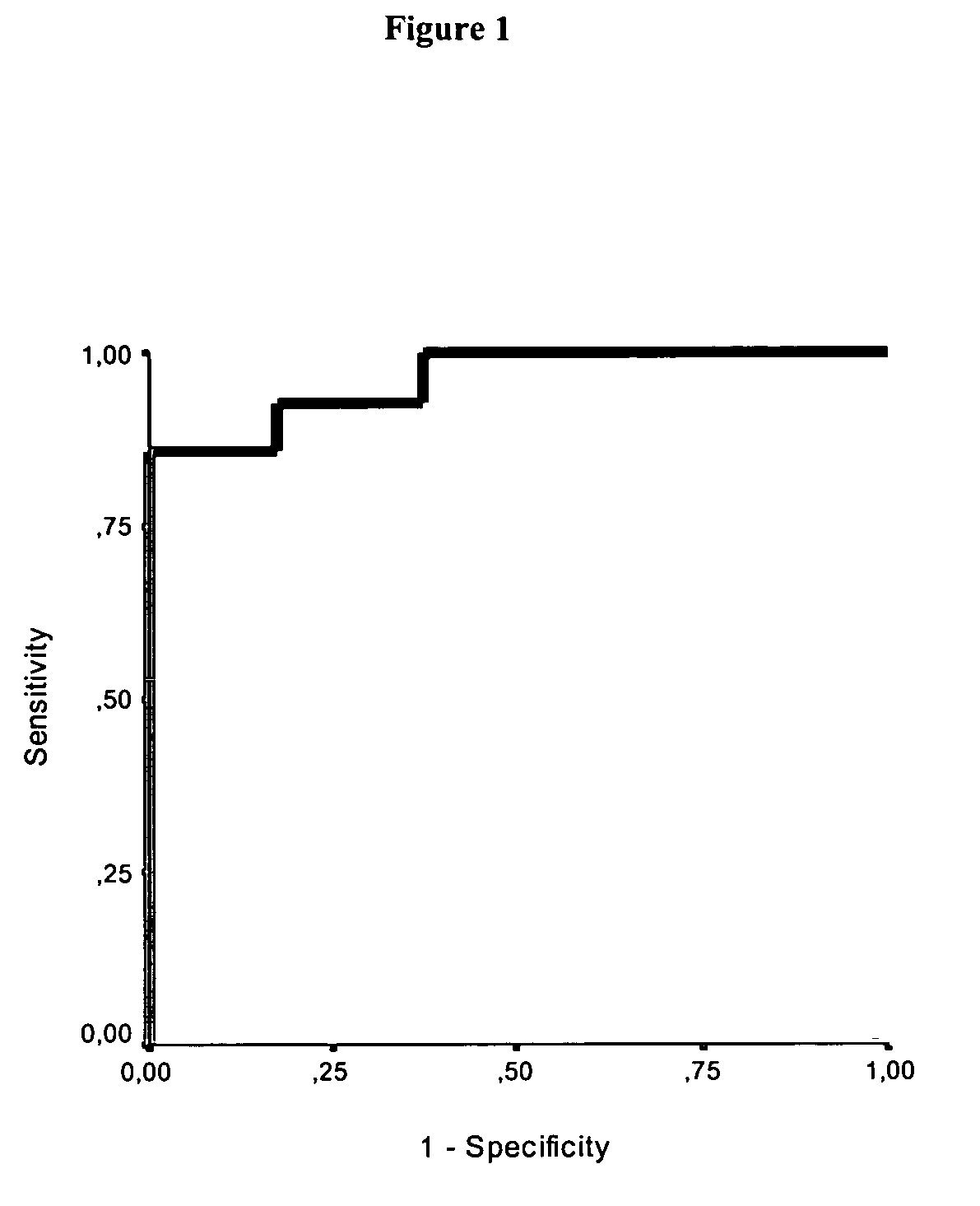
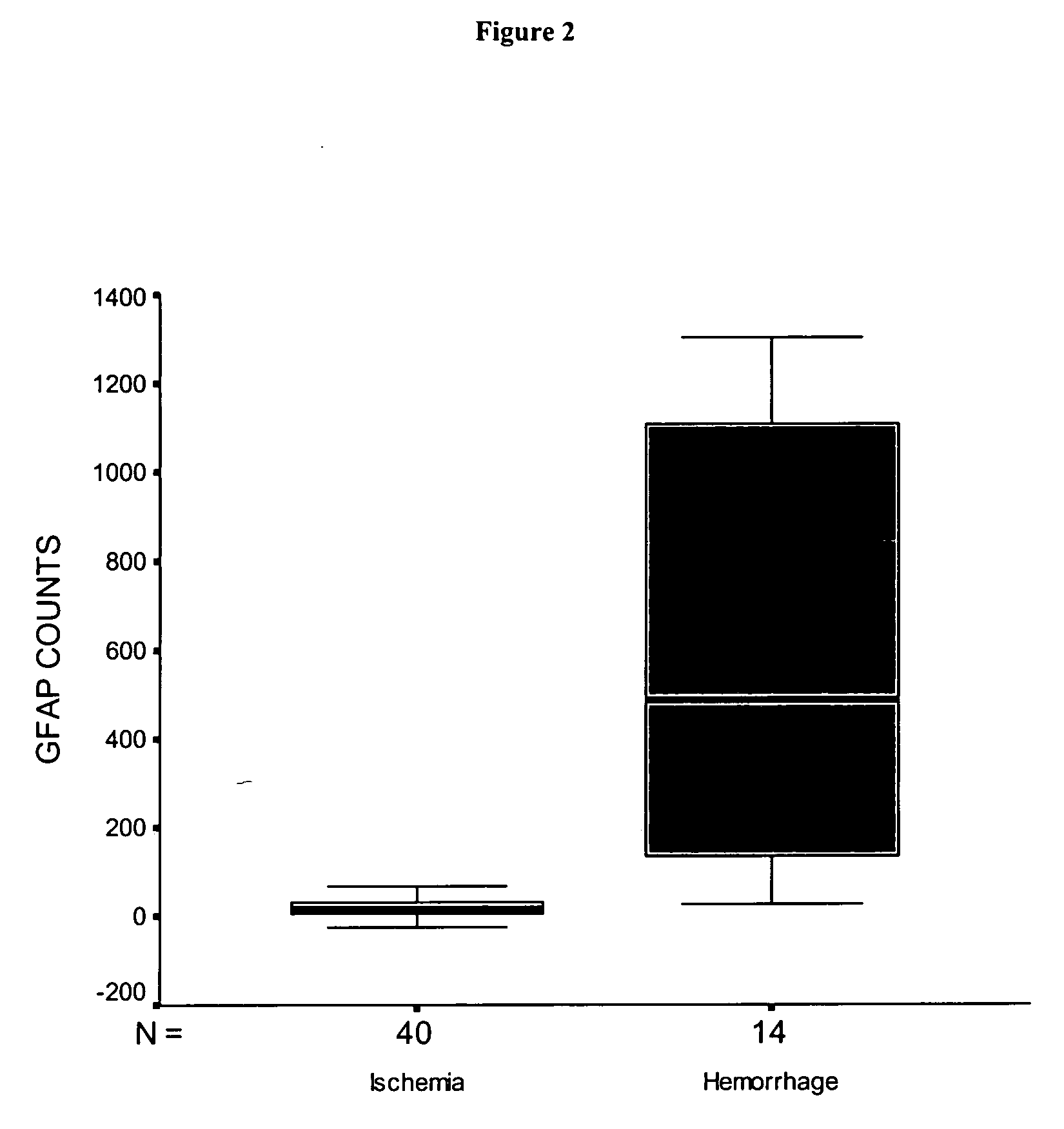
Use of GFAP for identification of intracerebral hemorrhage
a technology of intracerebral hemorrhage and gfap, which is applied in the field of intracerebral hemorrhage identification using gfap, can solve the problems of simultaneous expansion of cells in the penumbra, physical obstruction of arterial blood supply to the brain, and blockage or severe reduction of occlusion supply to the brain with oxygen and nutrients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific embodiments
EXAMPLE 1
Antibodies / Antibody Derivatives Used For Measurement of GFAP
[0082] With the exception of the blocking antibodies, all immunological reagents have been modified according to one of the following procedures. All antibodies used are commercially available, details / sources are given in detail below.
a) Biotinylation of Monoclonal Antibody MAB4A11 (Research Diagnostics Inc., Catalogue Number GFAPabm-411)
[0083] The purified antibody preparation comprising MAB4A11 was dialysed against the biotinylation buffer (50 mM KPO4, 100 mM NaCl, pH 8.5) and afterwards the solution was adjusted to a protein concentration of 0.5 mg / ml. D-biotinoyl-aminocaproic acid-N-hydroxysuccinimide ester was dissolved in DMSO and added to the antibody solution in a molar ratio of 1:5. The reaction was stopped by adding L-lysine and the surplus of the labelling reagent was removed by dialysis.
b) Ruthenylation of PABR-IgG, (DAKO-Cytomation, Catalogue-nr. ZO334)
[0084] The antibodies were dialysed agai...
example 2
Measurement of GFAP in a Patient Sample
[0086] Two assay formats have successfully been used in measurement of GFAP. Details are given below.
[0087] Wells of streptavidin-coated microtiter plates (RDG, catalogue nr. 148 705 1001) were incubated with 100 μl / well phosphate buffered saline with 0.05% Tween-20 (PBS-T) and with 1% bovine serum albumin (BSA) containing 1 μg / ml biotinylated IgG of MAB 4A11 (see Example 1a). Incubation was performed for 60 min at room temperature under shaking.
[0088] Wells were washed 4 times with the washing buffer PBS-T.
[0089] The incubation with native antigen in patient plasma, diluted in PBS-T buffer, was carried out with 100 μl / well for 1 hour at room temperature under shaking.
[0090] Wells were washed 4 times with the washing buffer PBS-T.
[0091] The incubation with PABR-IgG-digoxigenylated (cf. Example 1c) was performed with 100 μl / well PBS-T containing 8 μg / ml digoxigenylated PAB and 1% BSA for 1 hour at room temperatu...
example 3
[0105] Analytical and Clinical Data as Measured in the ELECSYS Assay:
TABLE 121-fold determination of a negative and a positive serum (blocked / normal format)SpecificsampletestVal 1Val 2Val 3Val 4Val 5Val 6Val 7UnitNumberAverageSTDEVCVcountspos. serumnormal2783271127062730262027492720Counts212719451.66%108427312730274527242772276027152719275327112671259127282730blocked1645166716301593163116811645Counts211635251.56%16111621161516531625159216591615162116131646168216501648neg. serumnormal1643164316411633162916381618Counts211630140.85%2716281642163916521611161316221593163116481622163116281628blocked1616161016251567162215831605Counts211603221.40%16021620162016211592159015631581164915991617156316111605
[0106]
TABLE 2Results as obtained with the GFAP calibrators usedStandards: CSF-Samplediluted in ELECSYSStd.Specific“Universaldiluent”Test formatVal 1Val 2NumberAverageDev.CVcounts1:50000 UD (169 pg / ml)blocked174317562175090.50%1:50000 UD (169 pg / ml)normal6109621226161731.18%44111:100000 UD (8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com