Adenoviral vectors for treating diseases
a technology of adenoviral vectors and diseases, applied in the field of gene therapy, can solve the problems of slow decline of human factor ix levels, ineffective second dose, and ineffective blocking of humoral effect of cyclosporin a
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Methods and Working Vectors
[0136] Methods for the construction and propagation of human adenovirus vectors are known in the art and will be understood to be applied in the Example presented below by the skilled practitioner of the art. Such would include the work of Hitt, M., et al Construction and propagation of human adenovirus vectors. In: Cell Biology: a Laboratory Handbook; J. Celis (Ed), Academic Press, N.Y. (1996); Graham, F. L. and Prevec, L. Adenovirus based expression vectors and recombinant vaccines. In: Vaccines: New Approaches to Immunological Problems. R. W. Ellis (ed) Butterworth. Pp. 363-390; and Graham, F. L. and Prevec, L. Manipulation of adenovirus vectors. In: Methods in Molecular Biology, Vol. 7: Gene Transfer and Expression Techniques. E. J. Murray and J. M. Walker (eds) Humana Press Inc., Clifton, N.J. pp 109-128, 1991. The materials and methods described in these articles were used below. See also, Hermiston, T. et al., Methods in Molecular Medicine...
example 2
Construction of E3 Shuttle Vectors
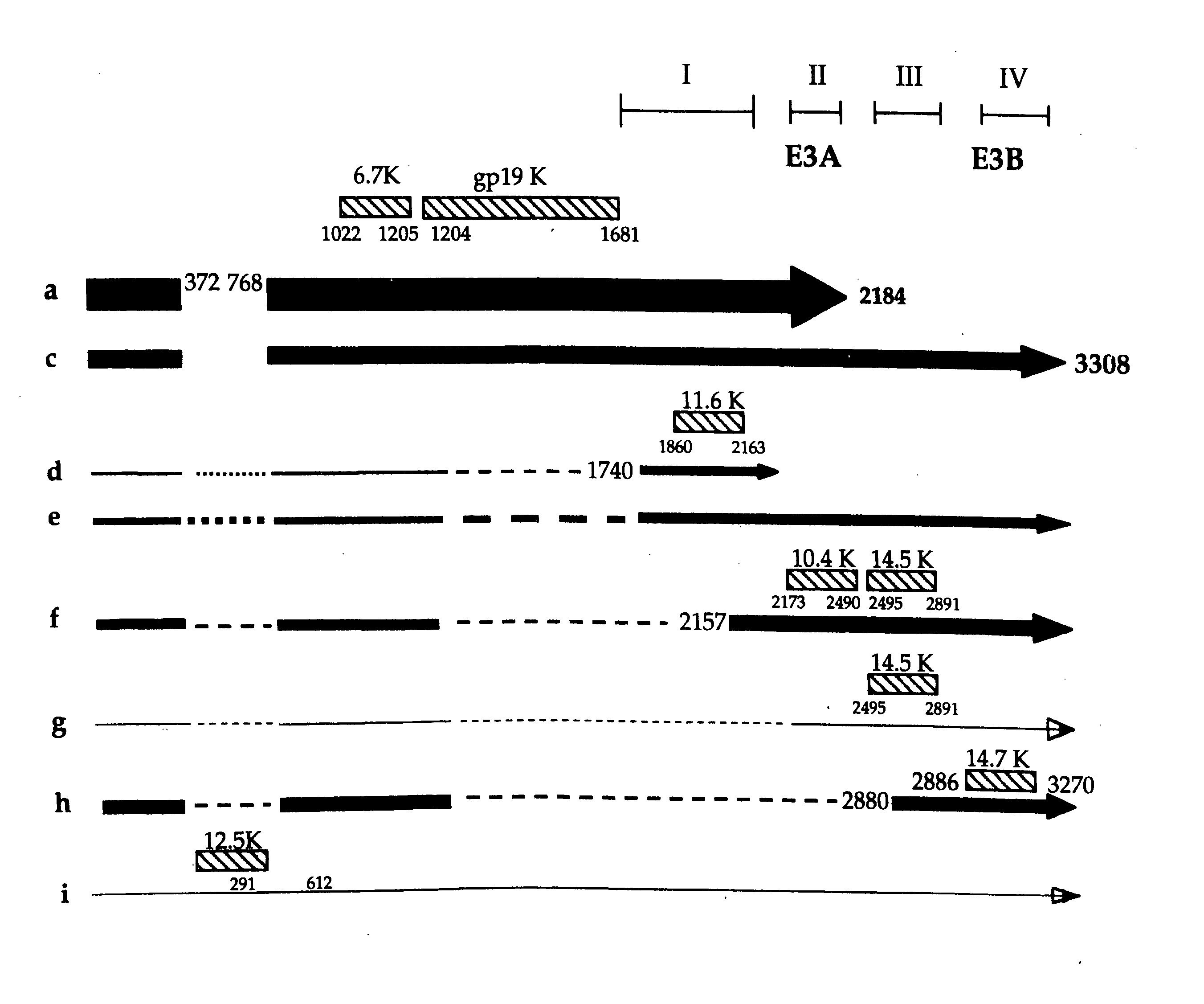
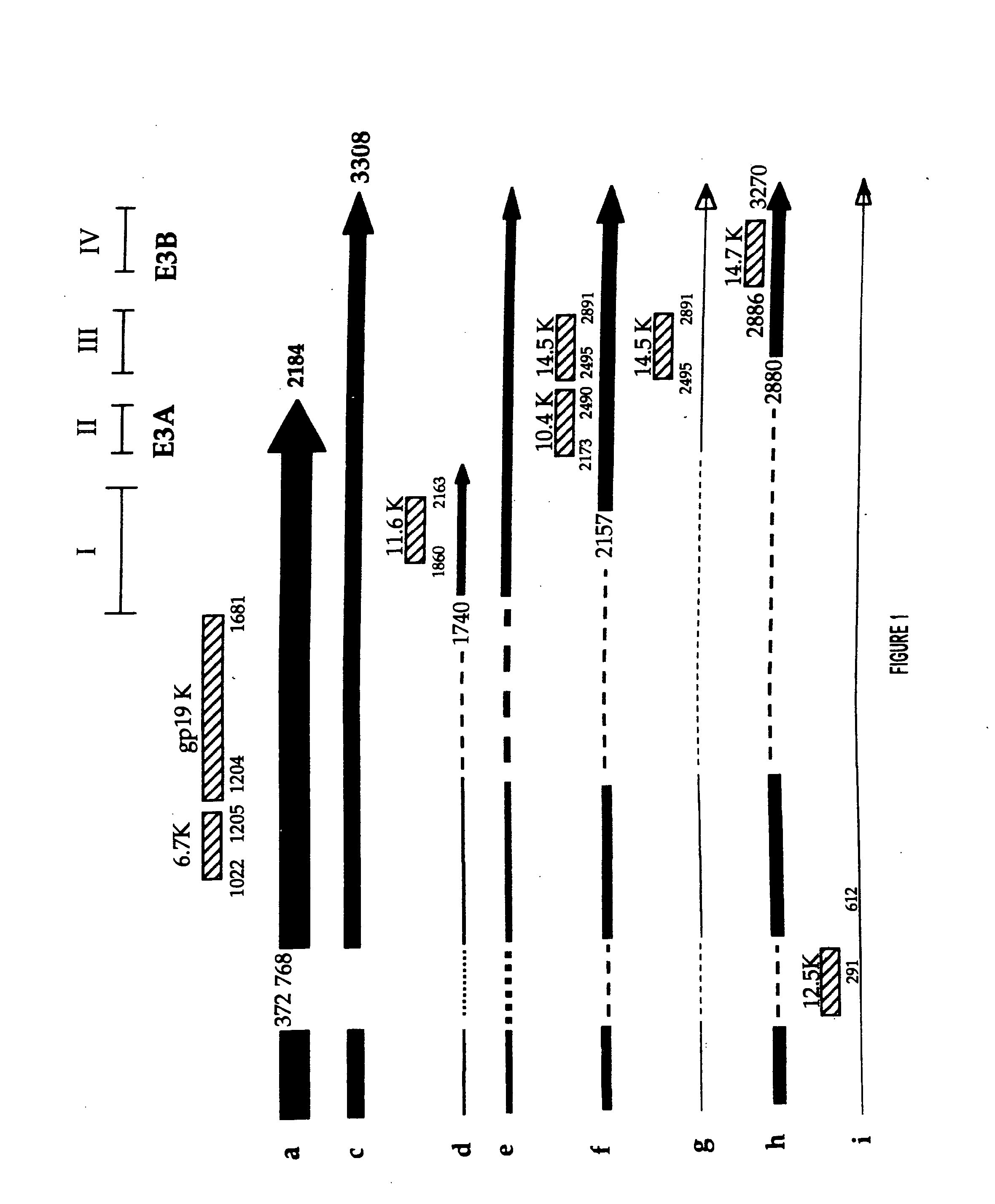
[0140] Using the above vectors, the following restrictions sites were engineered into the E3 region of adenovirus 5: PacI, ClaI, PmeI, SwaI, BamHI, BstBI, SspI, NheI, and StuI and EcoRV. Their relative positions in the E3 region are shown in FIG. 7. The restriction sites were carefully positioned so as to not knowingly disrupt, or minimally disrupt, critical splicing and polyadenylation signals (see, FIG. 1). Also considered was the coding sequence of proteins; in most cases, the coded amino acid was not changed, and when changes had to be made, they were conservative.
[0141] Because of the position of the engineered sites, some mutations had to be performed sequentially. All of the oligonucleotide sequences used for mutagenesis and the exact location (position number in Ad5) are listed in the tables. All mutations were confirmed by restriction digests and all constructs were sequenced. Table 2 summarizes the restriction sites that were added to t...
example 3
Construction of Virus Controls and Optional Plasmids for Virus Production
[0149] For controls, each of the E3 genes was deleted using the engineered sites. To do this, the shuttle plasmids were cut with the following pairs of enzymes, filled in using T4 DNA polymerase, and religated: PacI and PmeI, SunI and MunI, NheI and PmeI, BstBI and StuI (all in pG-E3SV), ClaI and SwaI (in pG-E3SV+V), ClaI and SspI (in pG-E3SV+V), and BamHI and SwaI (in pG-E3SV+B).
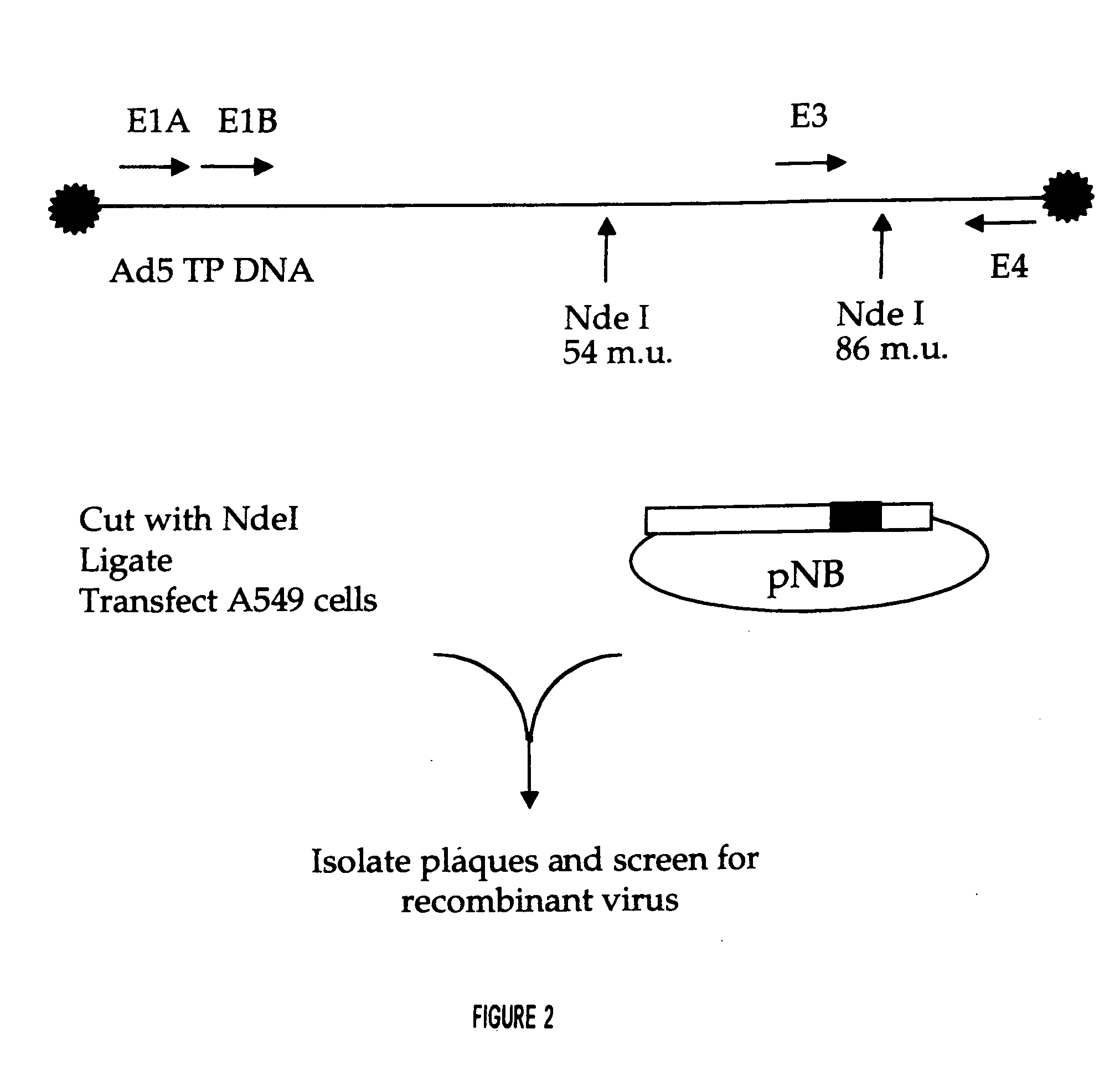
[0150] In addition to the pG plasmids referred to above that were used to generate the invention viruses, FIG. 2 shows another plasmid that could also be used, termed pNB. The pNB has two SpeI sites: one in the Ad5 insert and one in the pGEM5 MCS. The fragment from pNB which contained a portion of the MCS and NdeI 19549 to SpeI 27082 was inserted into the SpeI-cut pG-PPCS plasmid. The orientation was confirmed to be correct, and the resulting plasmid termed pNB-PPCS. All of the final versions of the E3 shuttle vectors were cloned in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com