Use of substituted-1, 5-dideoxy-1, 5-imino-D-glucitol compounds for treating hepatitis virus infections
a technology of hepatitis virus infection and substituted compounds, which is applied in the field of methods, can solve the problems of unpredictability, inability to treat these often lethal diseases, and inability to achieve treatment. treatment, prevent the development of drug resistance, and reduce the toxicity of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1,5-(butylimino)-1,5-dideoxy-D-glucitol
[0310] A solution of 1,5-dideoxy-1,5-imino-D-glucitol (5.14 g, 0.0315 mole), butyraldehyde (3.35 ml, 0.0380 mole) and Pd black (1 g) in 200 ml methanol was hydrogenated (60 psi / 29C / 21 hrs.). After filtering the resulting mixture, the filtrate was concentrated in vacuo to an oil. The title compound was crystallized from acetone, and recrystallized from methanol / acetone, m.p. ca. 132C. The structure assignment was supported by NMR, infrared spectra and elemental analysis.
[0311] Analysis calcd. for C10H21NO4: C, 54.78; H, 9.65; N, 6.39. Found: C, 54.46; H, 9.33; N, 6.46.
example 2
Preparation of 1,5-(butylimino)-1,5-dideoxy-D-glucitol, tetraacetate
[0312] Acetic anhydride (1.08 g, 0.0106 mole) was added to the title compound of Example 1 (0.50 g, 0.0023 mole) in 5 ml pyridine and stirred for 17 days at room temperature. The product was evaporated under nitrogen gas. The resulting title compound was purified by silica gel chromatography. Structure assignment was supported by NMR, infrared spectra and elemental analysis.
[0313] Analysis calcd. for C18H29NO8: C, 55.80; H, 7.54; N, 3.62. Found: C, 55.42; H, 7.50; N, 3.72.
example 3
Anti-Hepatitis B Virus Activity of Various N-Substituted-1,5-Dideoxy-1,5-Imino-D-Glucitol Compounds In Vitro
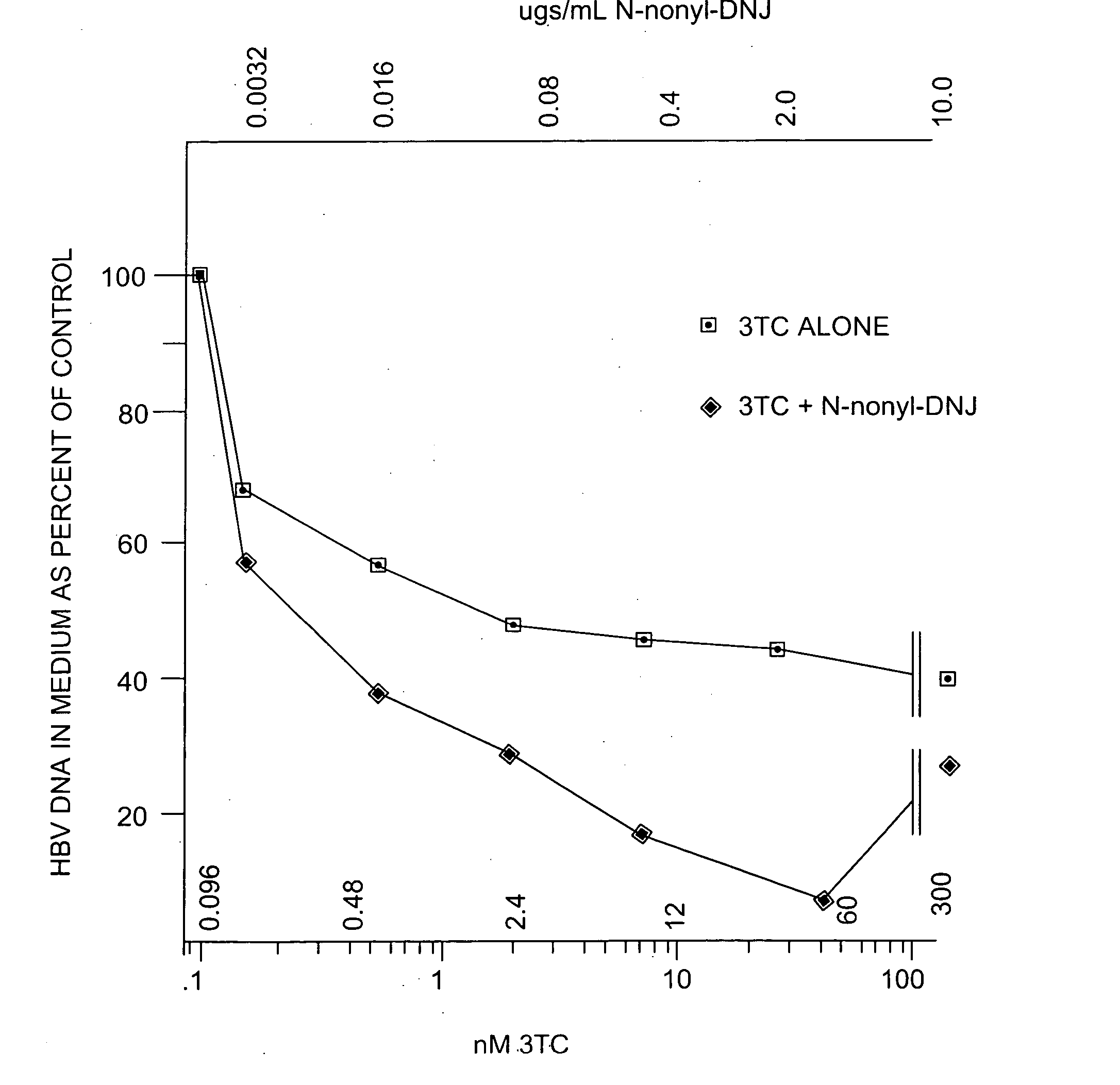
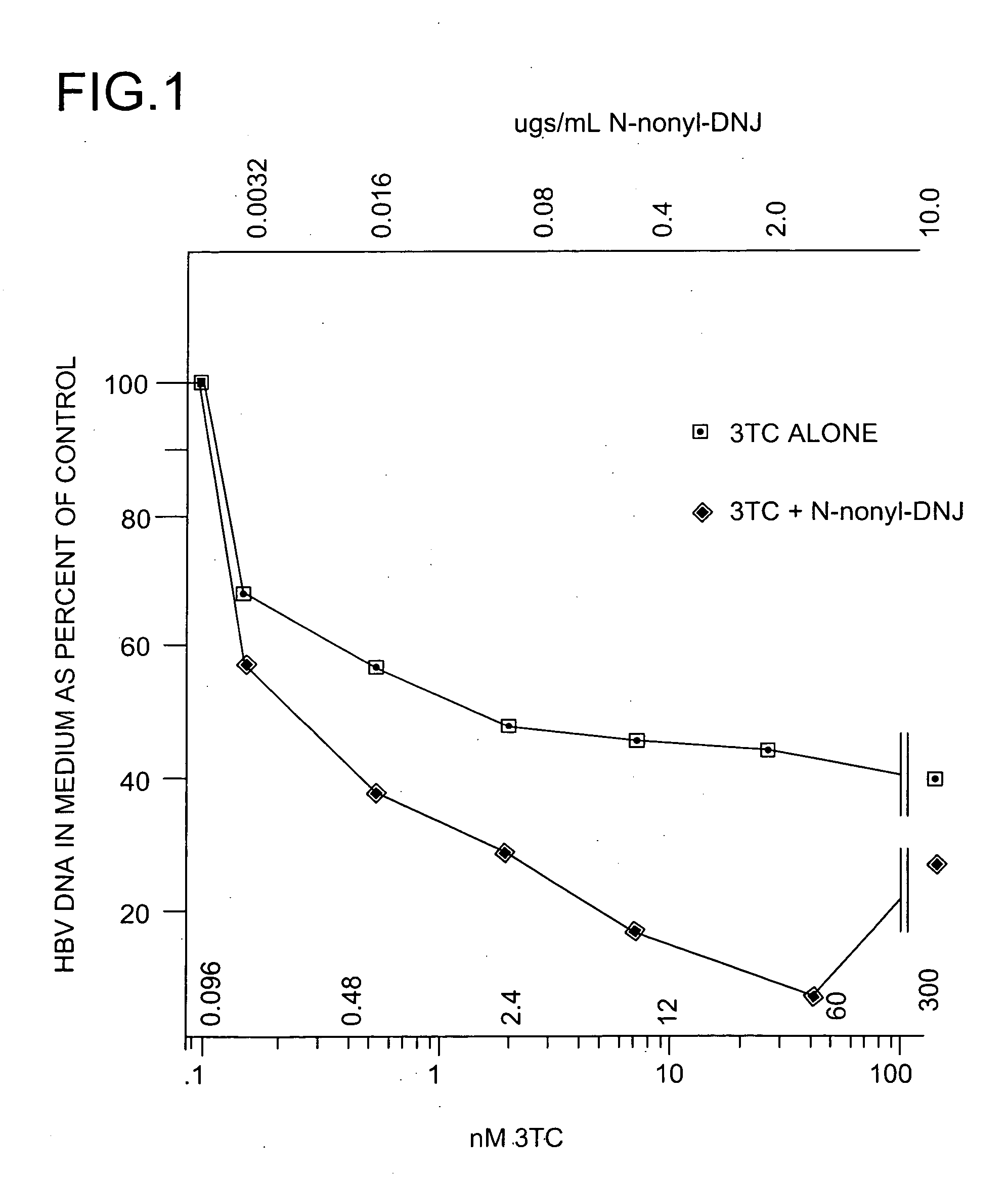
[0314] The anti-hepatitis B virus activity and effect on cell viability of a number of different N-substituted-1,5-dideoxy-1,5-imino-D-glucitol compounds were assessed using an in vitro assay employing chronically hepatitis B virus secreting HepG2.2.15 cells. The method employed was essentially that described in Block et al. (1994) Proc. Natl. Acad. Sci. USA 91:2235-2239. The results are shown in Tables 2 and 3.
TABLE 2Effect of N-Substituted-1,5-Dideoxy-1,5-Imino-D-GlucitolCompounds on Hepatitis B Virus Secretion and Viability ofHepG2.2.15 CellsRelative amountofHBV secretedCompound and% ViableAs A %[Concentration]1+ / −1 S.D.2,5of Control3Control90 + / − 7 (n = 4)100NBDNJ[200]94 + / − 6 (n = 10) 37.0 + / − 13 (n = 15)NBDNJ[1000]88 + / − 8 (n = 10) 3.2 + / − 5 (n = 15)1 [200]90 + / − 2 (n = 4)85.0 + / − 5 (n = 8) 1 [1000]87 + / − 3 (n = 4)35.0 + / − 6 (n = 8) 2 [200]90 + / − 6 (n = 4) 107.0 + / − 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com