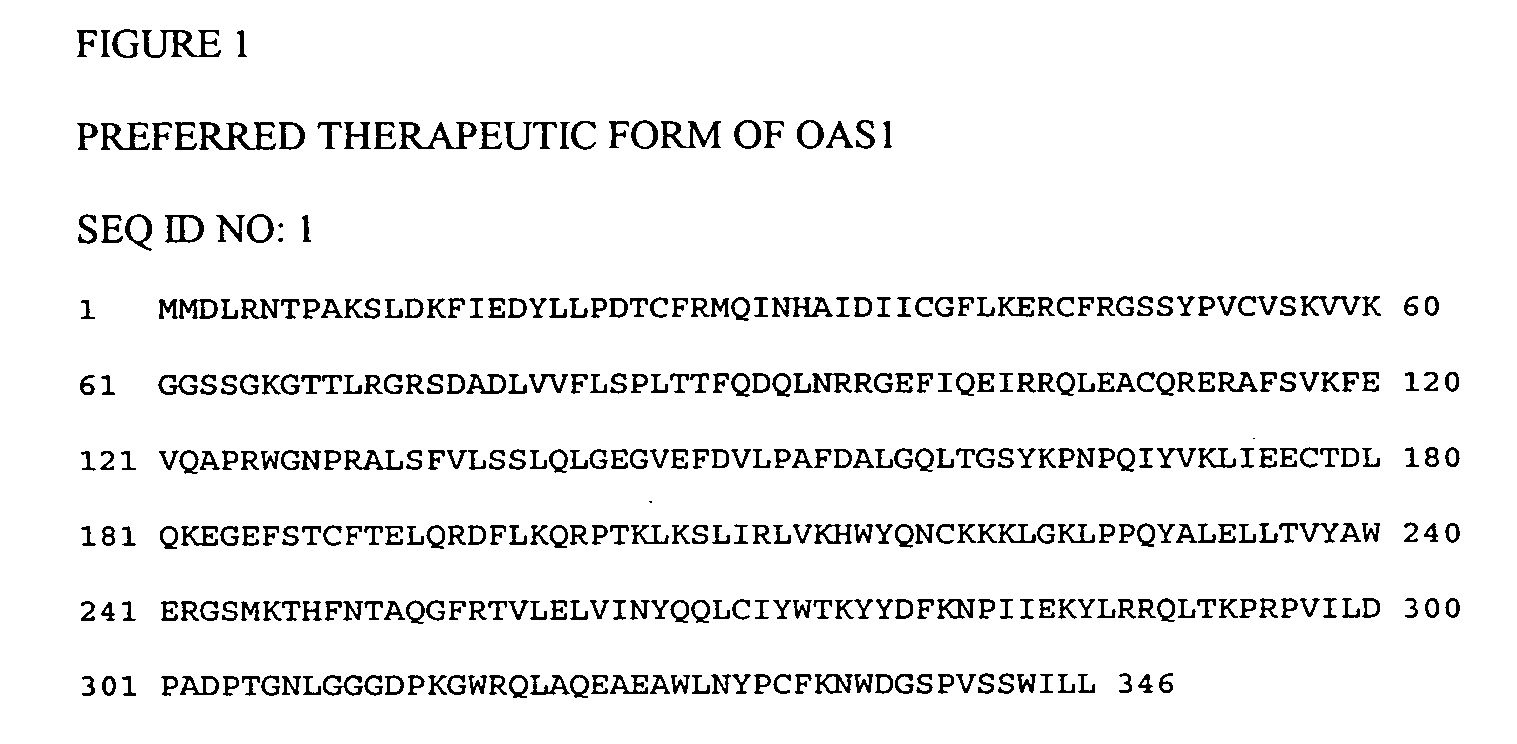
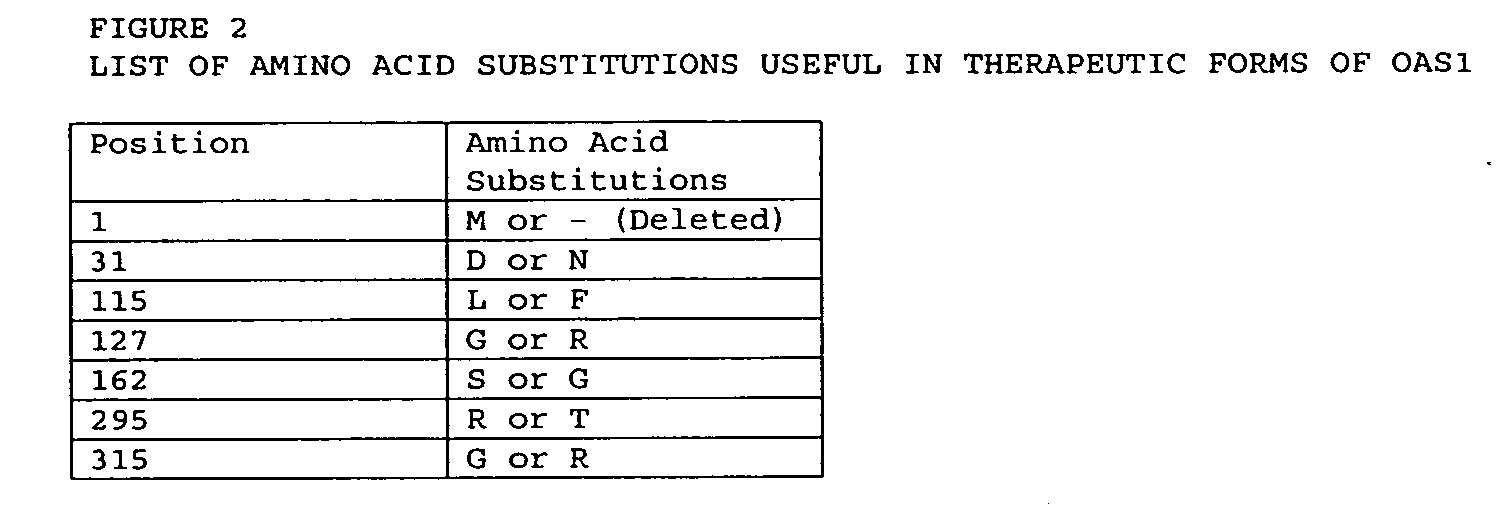
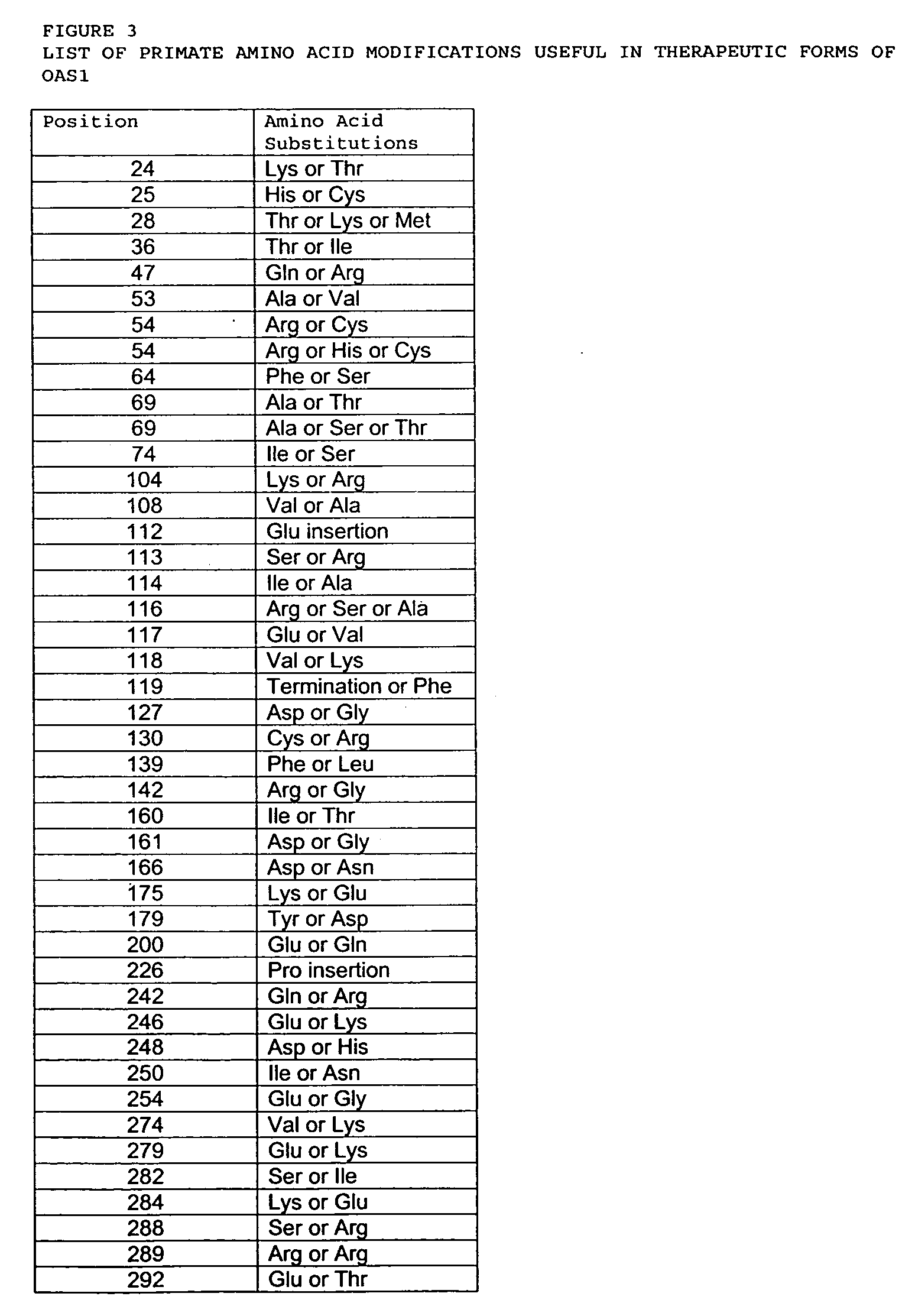
Mutations in OAS1 genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Amino Acid Modifications in Non-human Primate OAS1 Proteins
[0201] OAS1 genes from non-human primates were sequenced and compared with mutations found in the human OAS1 gene. Such mutations provide additional insight into evolution of the OAS1 gene and protein. Evolutionarily conserved amino acids suggest sites important, or critical, for OAS1 function or enzymatic activity. Conversely, OAS1 amino acid sites that have recently mutated, for example in humans only, or show a plurality of amino acid substitutions across primates, indicate sites less critical to function or enzymatic activity. The abundance of mutated sites within a particular motif of the OAS1 protein are correlated with the tolerance of that functional domain to modification. Such sites and motifs are optimized to improve protein function or specific activity. Similarly, mutations in genes and proteins with immune or viral defense functions like OAS1 are hypothesized to result from historical challenge by viral infect...
example 2
Preparation and Sequencing of cDNA
[0204] Total cellular RNA is purified from cultured lymphoblasts or fibroblasts from the patients having the hepatitis C resistance phenotype. The purification procedure is performed as described by Chomczynski, et al., Anal. Biochem., 162:156-159 (1987). The cells are homogenized in 10 milliliters (ml) of a denaturing solution containing 4.0M guanidine thiocyanate, 0.1M Tris-HCl at pH 7.5, and 0.1M beta-mercaptoethanol to form a cell lysate. Sodium lauryl sarcosinate is then admixed to a final concentration of 0.5% to the cell lysate after which the admixture was centrifuged at 5000×g for 10 minutes at room temperature. The resultant supernatant containing the total RNA is layered onto a cushion of 5.7M cesium chloride and 0.01M EDTA at pH 7.5 and is pelleted by centrifugation. The resultant RNA pellet is dissolved in a solution of 10 mM Tris-HCl at pH 7.6 and 1 mM EDTA (TE) containing 0.1% sodium docecyl sulfate (SDS). After phenolchloroform extr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com