Two-component pharmaceutical composition for the treatment of pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
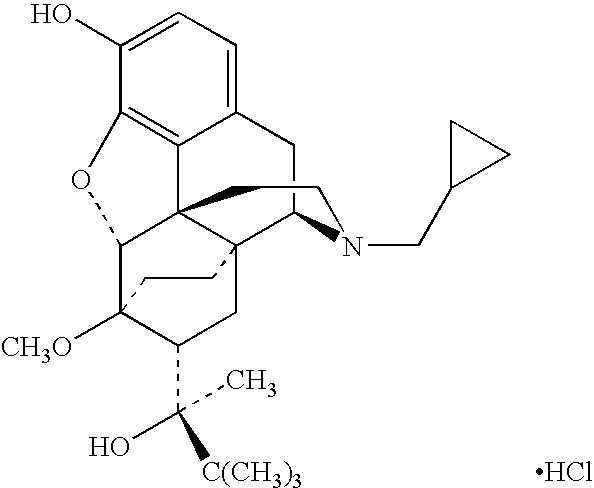
[0037] Patients taking 15-80 mg / day of hydrocodone or 40-320 mg / day of oxycontin were switched to a pharmaceutical dosage unit including 2.5 mg piroxicam and 2 mg buprenorphine in a sublingual tablet. The sublingual tablets were administered to each patient twice daily. The patients perceived pain was measured using a visual analog scale (VAS).
[0038] Patients formerly taking 15-80 mg / day of hydrocodone experienced a 21% decrease in perceived pain scores after conversion. The perceived pain scores also remained stable when ½ dose reduction was attempted after a 22 day stabilization phase.
[0039] Patients that had been taking 40-320 mg / day of oxycontin experienced a 27% reduction in perceived pain 72 hours after conversion. No withdrawal or parasympathetic symptoms were noted in the oxycontin patients. The perceived pain scores remained stable when dose reduction was attempted after a 40-day stabilization phase.
[0040] The patients also generally reported feelings of being more in co...
example 2
[0041] Patients taking 20-140 mg / day of methadone were also switched to a pharmaceutical dosage unit including 2.5 mg piroxicam and 2 mg buprenorphine in a sublingual tablet. These patients took an average of 6 days to convert before withdrawal was completely eliminated.
[0042] Patients taking 20-50 mg / day of methadone converted without ill effects, but did not take methadone the first day.
[0043] Patients taking 50-140 mg / day of methadone were first titrated down with Ultram (2-3 tablets every 4 hrs) for four days. Following this period, patients were given injections containing 0.03 mg buprenorphine and 60 mg torodol to see if further time was needed before the sublingual tablets containing buprenorphine and piroxicam could be started without risk of precipitating withdrawal. Significant withdrawal was observed in 18% of the patients on the first day the sublingual tablets were administered. Stomach tightness, nausea, and restless legs were the most common complaints from these pa...
example 3
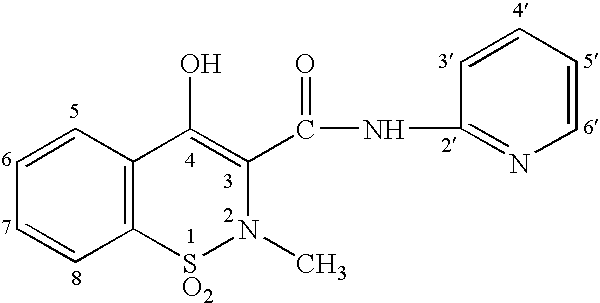
[0045] Patients that had been taking piroxicam were also switched to a pharmaceutical dosage unit including 2.5 mg piroxicam and 2 mg buprenorphine in a sublingual tablet. The patients reported a 50% reduction in GI upset compared to when they were taking piroxicam orally.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com