Immediate release pharmaceutical granule compositions and a continuous process for making them
a technology of compositions and pharmaceutical granules, applied in the direction of pharmaceutical delivery mechanisms, pill delivery, medical preparations, etc., can solve the problems of affecting patient compliance, capsules may be too small, and the latter is rather difficult to manufactur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Twin Screw Extruder for Producing a Pharmaceutical Granule Composition
[0088] The twin screw extruder used for performing the following pharmaceutical granule preparations is described in FIG. 3. It consists of seven distinct zones, wherein zones (1), (2), (4) and (6) are three transport zones, zones (3) and (5) are two mixing zones and zone (7) is a densification zone (which could alternatively be omitted, if desired). The extruder is placed within a granulation chamber provided with inlets for supplying the drug and the various excipients.
examples 2 and 3
Pharmaceutical Granule Formulations Including a Malto-Dextrin and Xanthan Gum
[0089] The following formulations were prepared using the extruding equipment of example 1:
Low water-soluble drug:100gPolyethyleneglycol 400:52.5gPolyethyleneglycol 4000:187.5gMaltodextrin 01982622.5gXanthan gum:37.5g
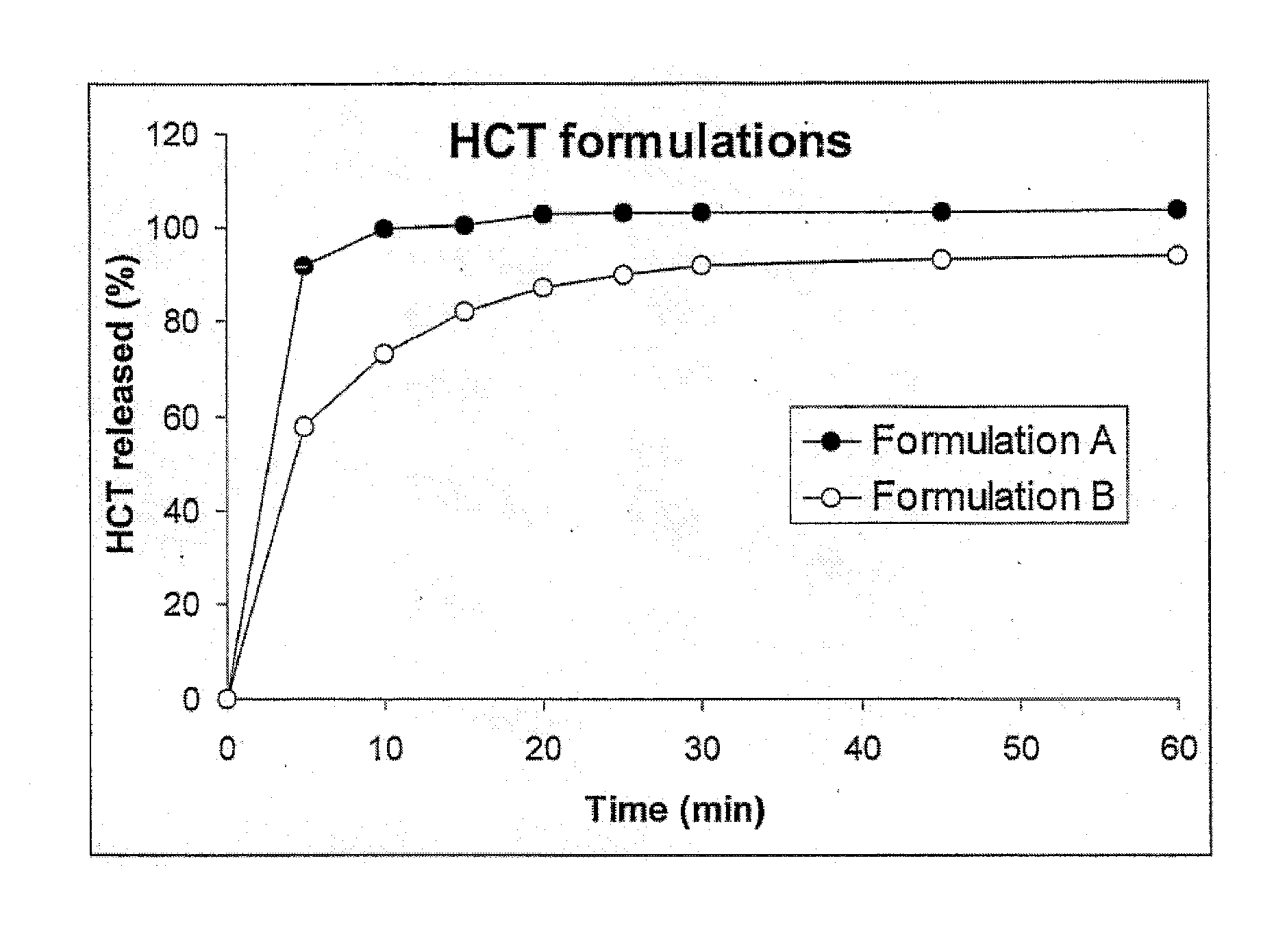
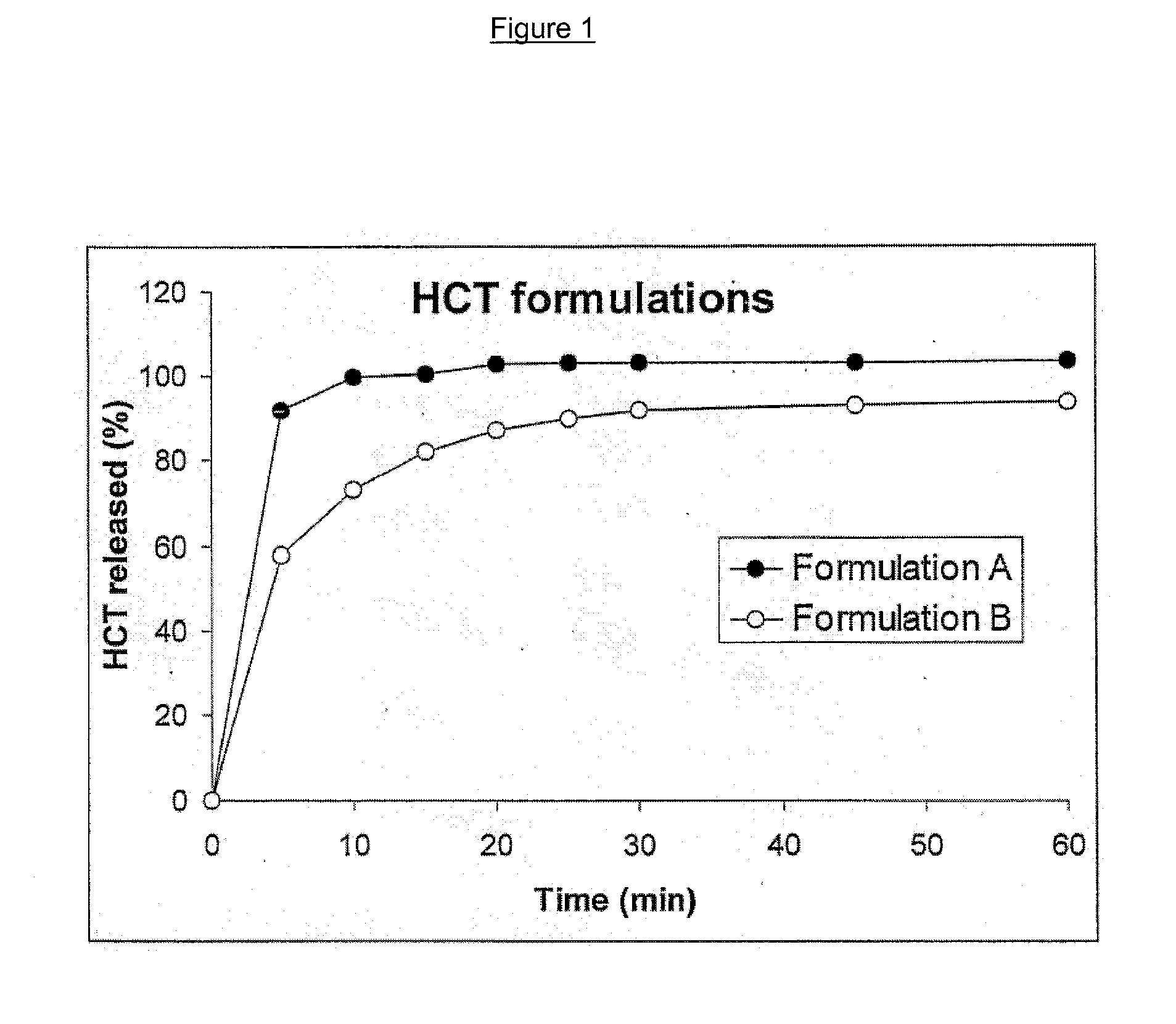
[0090] Maltodextrin 01982 is a neutral taste, medium DE maltodextrin with good dispersibility which complies with European and U.S. Pharmacopeia and which is commercially available from Cerestar (Neuilly-sur-Seine, France). The solid fraction of the formulation consisting of hydrochlorothiazide (example 2), PEG 4000, maltodextrin and xanthan gum was homogenised in a planetary mixer. This mixture was fed into the twin screw extruder at a rate of 29.9 g / min. The liquid phase (PEG400) was continuously pumped into the twin screw extruder at a rate of 6.9 g / min. The screw speed during the extrusion was 250 rpm. The temperature of the different zones of the twin screw extruder was set at 25° C., y...
examples 4 and 5
Pharmaceutical Granule Formulations Including Micro-Crystalline Cellulose
[0093] The following formulations were prepared using the extruding equipment of example 1:
Low water-soluble drug:100gPolyethyleneglycol 400:52.5gPolyethyleneglycol 4000:250gAvicel PH 101:298.75gAvicel CL611:298.75g
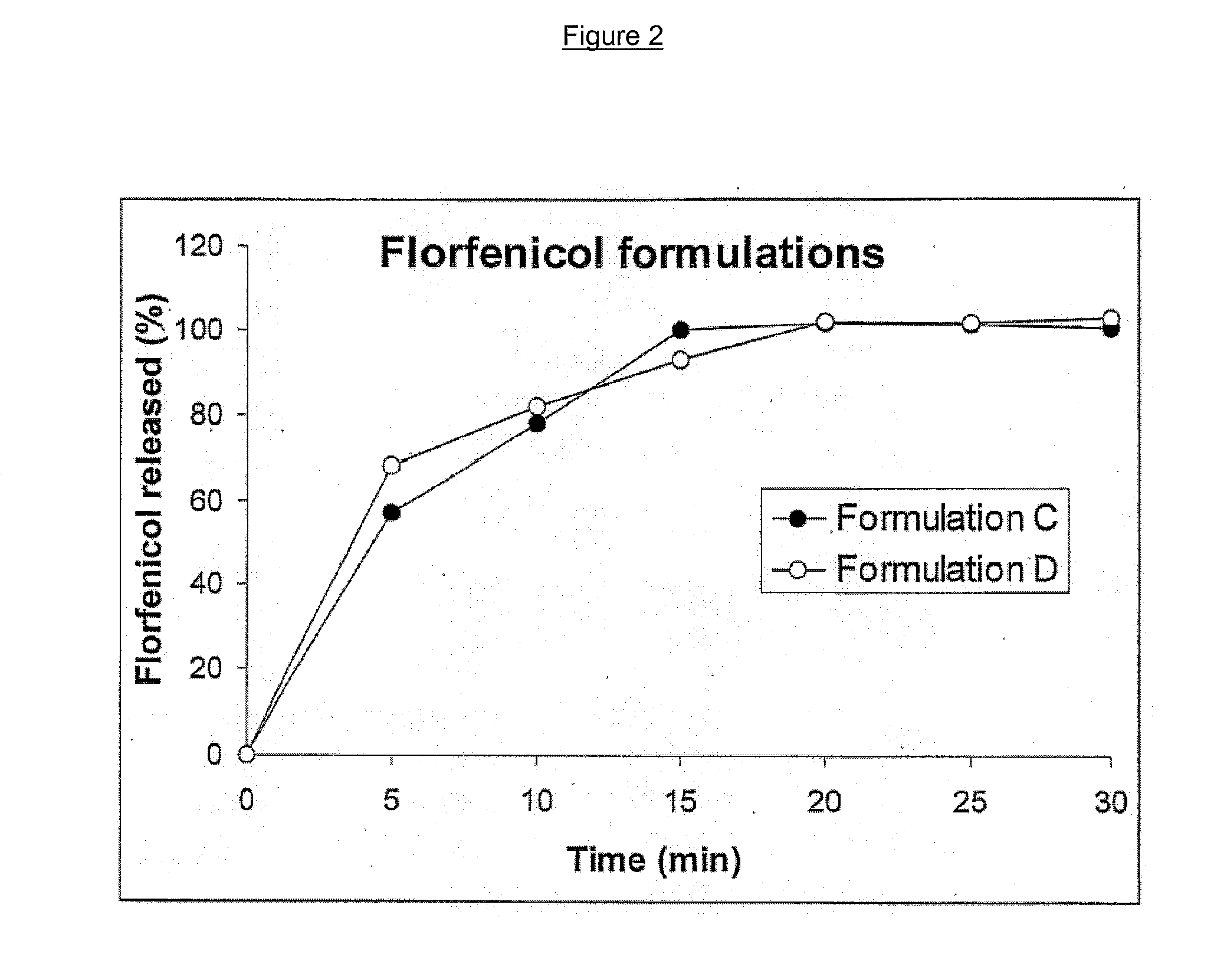
[0094] The solid fraction of the formulation consisting of hydrochlorothiazide (example 4), PEG 4000, Avicel PH 101 / Avicel CL 611 (commercially available from FMC Corporation, Philadelphia, Pa.) was homogenised in a planetary mixer. The homogeneous mixture was then fed into the twin screw extruder at a rate of 27.6 g / min. The liquid phase (PEG 400) was continuously pumped into the twin screw extruder at a rate of 9.2 g / minute. The screw speed during the extrusion was 250 rpm. The temperature of the different zones of the twin screw extruder was set at 25° C. yielding experimental temperatures of 25° C., 28° C., 27° C., 26° C. and 25° C. in zones 1 to 5, respectively.
[0095] In the case of florfeni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water-solubility | aaaaa | aaaaa |
| water-solubility | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com