Reinforced matrices
a matrix and reinforcement technology, applied in the field of reinforcement matrix, can solve the problems of cartilage matrix stiffness reduction, cartilage matrix injury, and matrix rupture,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0052] Chondrocytes were grown in minimal essential culture medium containing HAM F12, 15 mM Hepes buffer and 5 to 7.5% autologous serum in a CO2 incubator at 37° C. and handled in a Class 100 laboratory at Verigen Transplantation Service ApS, Copenhagen, DK. Other compositions of culture medium may be used for culturing the chondrocytes.
[0053] The cells were trypsinised using trypsin EDTA for 5 to 10 minutes and counted using Trypan Blue viability staining in a Bürker-Türk chamber. The cell count was adjusted to 7.5×105 cells per ml. One NUNCLON™ plate was uncovered in the Class 100 laboratory.
[0054] Six Pieces of a size of 1 cm2 each of commercially available collagen I / III fleece (Chondro-Gide®, Geistlich, CH) were placed under aseptic conditions into the bottom of the well in the NUNCLON™ cell culture tray.
[0055] Approximately 5×106 of the chondrocytes in 5 ml of the culture medium were placed directly on top of the carrier material and dispersed over the surface. The plate w...
example 2
[0059] Chondrocytes were grown in minimal essential culture medium containing HAM F12, 15 mM Hepes buffer and 5 to 7.5% autologous serum in a CO2 incubator at 37° C. and handled in a Class 100 laboratory at Verigen Transplantation Service ApS, Copenhagen, DK. Other compositions of culture medium may be used for culturing the chondrocytes.
[0060] The cells were trypsinised using trypsin EDTA for 5 to 10 minutes and counted using Trypan Blue viability staining in a Bürker-Türk chamber. The cell count was adjusted to 7.5×105 cells per ml. One NUNCLON™ plate was uncovered in the Class 100 laboratory.
[0061] Six Pieces of a size of 1 cm2 each of a collagen I / III matrix (Immedex, France) was cut to a suitable size fitting into the bottom of the well in the NUNCLON™ cell culture tray and placed under aseptic conditions on the bottom of the well.
[0062] Approximately 5×105 of the chondrocytes in 5 ml of culture medium were placed directly on top of the carrier material and dispersed over th...
example 3
[0065] Six pieces, 1 cm2 each in size, of the collagen I / III matrix of Example 2 were incubated for 2 hours at a temperature of 50° C. under gentle stirring with a solution of soluble elastin (EPC Inc., USA) in a suitable buffer such as phosphate buffer (0.02M KH2PO4, pH 7.4) and the pH value was then brought down to 5.0 by adding acetic acid under gentle stirring. The coacervation reaction was allowed to occur for 5 hours.
[0066] The suspension was then lyophilized at a temperature of 25° C. and a pressure of 0.05 mbar.
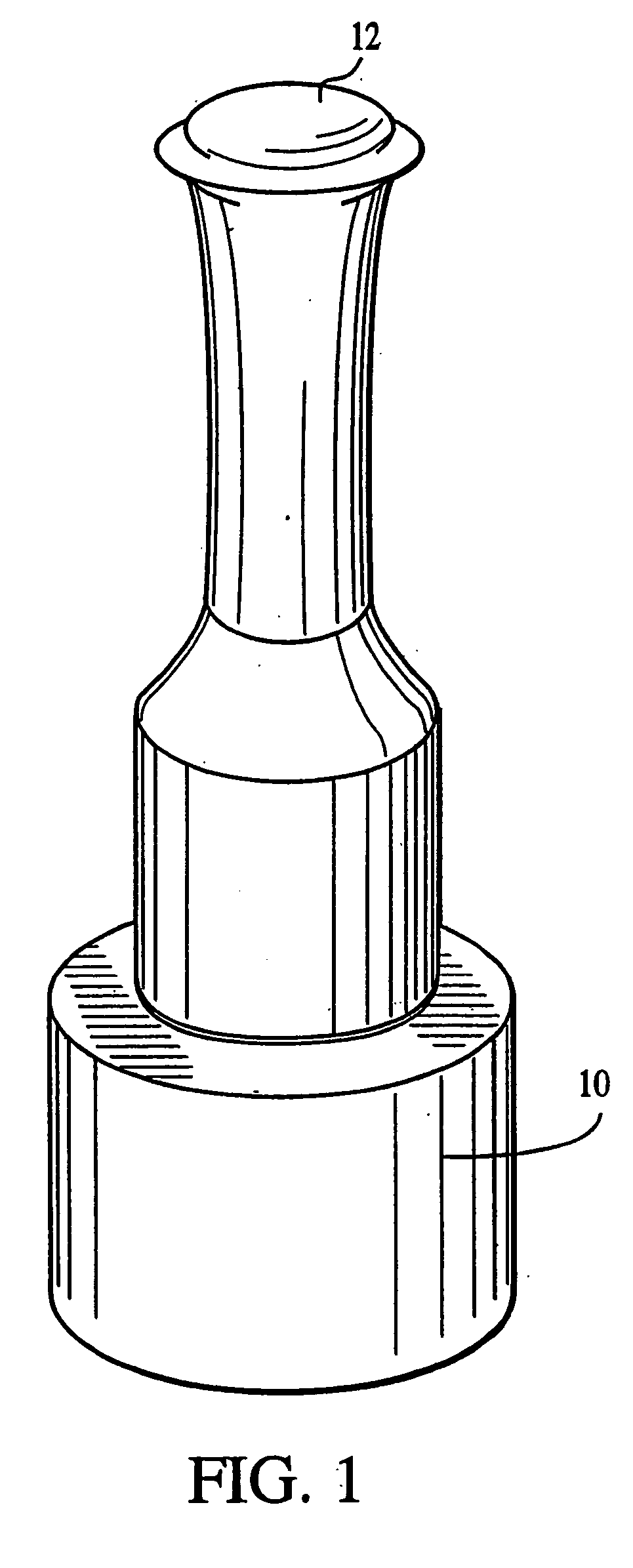
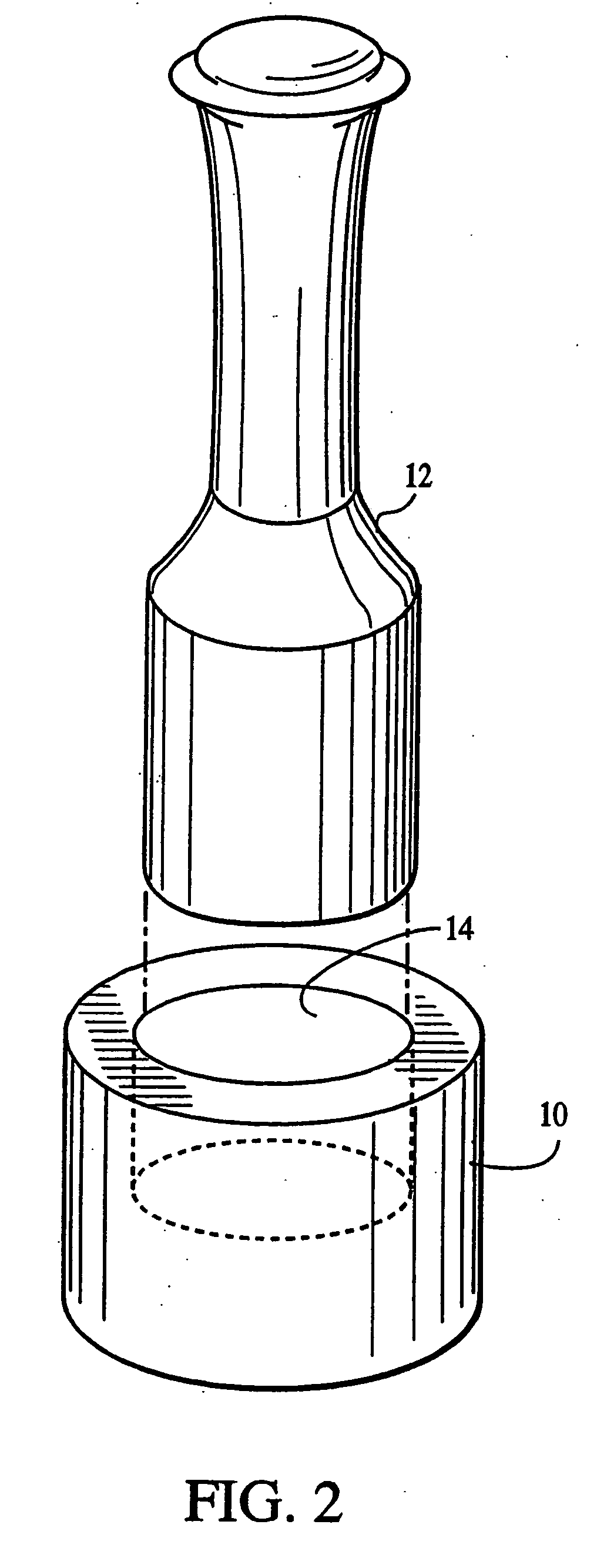
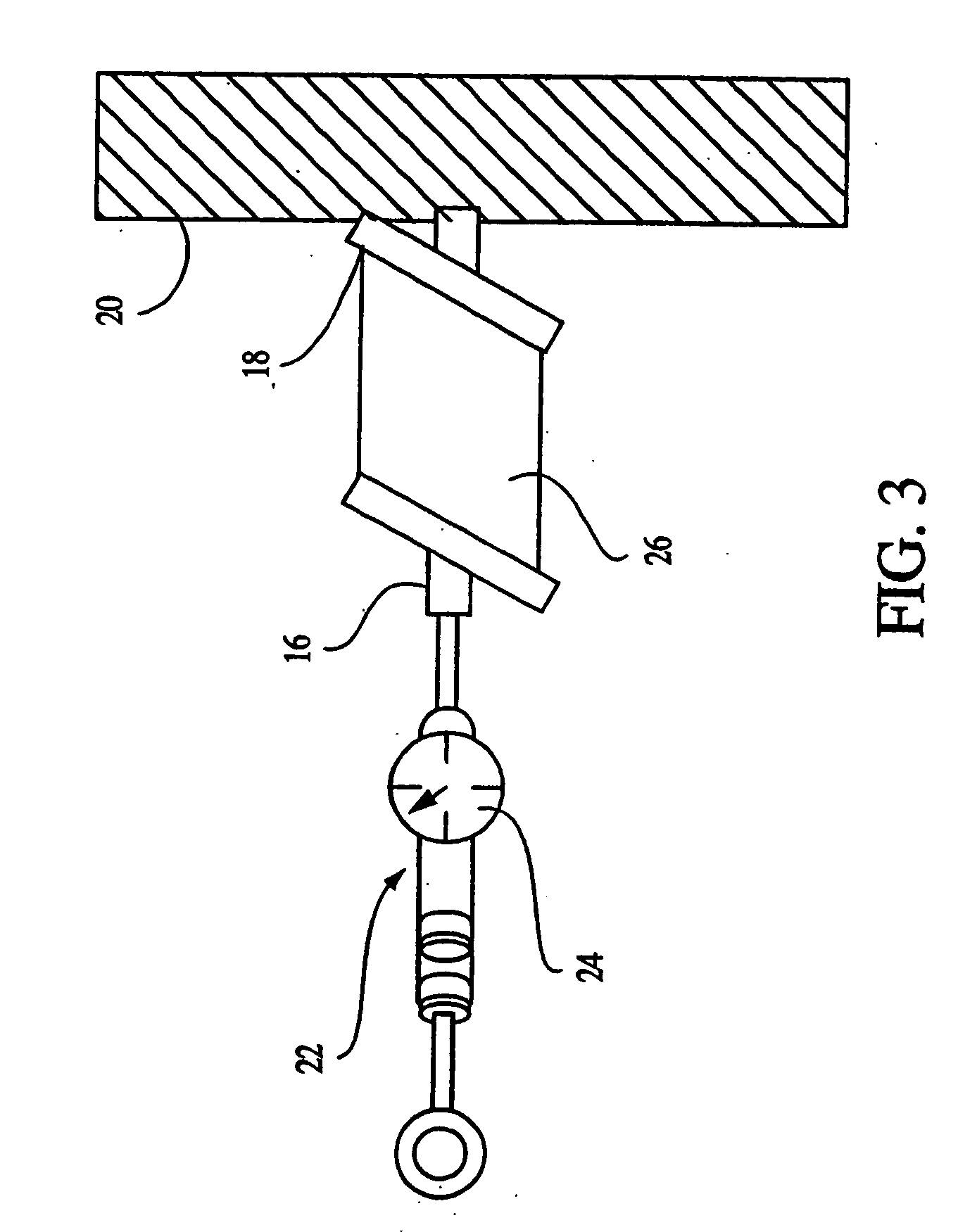
[0067] The lyophilization yielded a fleece-like material which was pressed mechanically using the apparatus in FIGS. 1 and 2 into sheets for use with cells as an implantation article. The material was pressed for about 24 hours until a sheet-like material which resisted tearing upon being handled was obtained.
[0068] Six Pieces, each 1 cm2 in size, of the fleece matrix were cut to a suitable size fitting into the bottom of the well in the NUNCLON™ cell culture tray ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com