Renal progenitor cells from embryonic stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
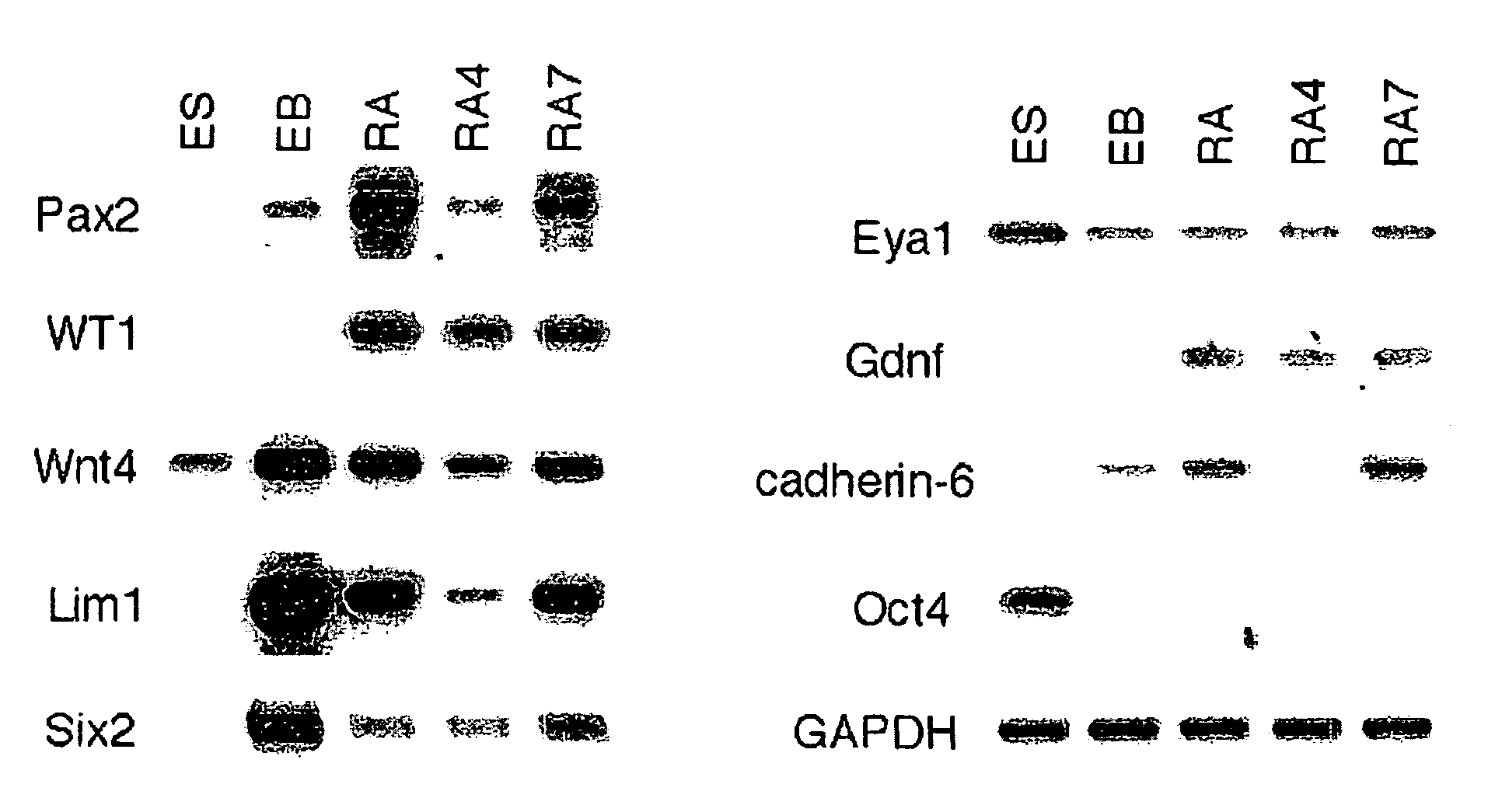
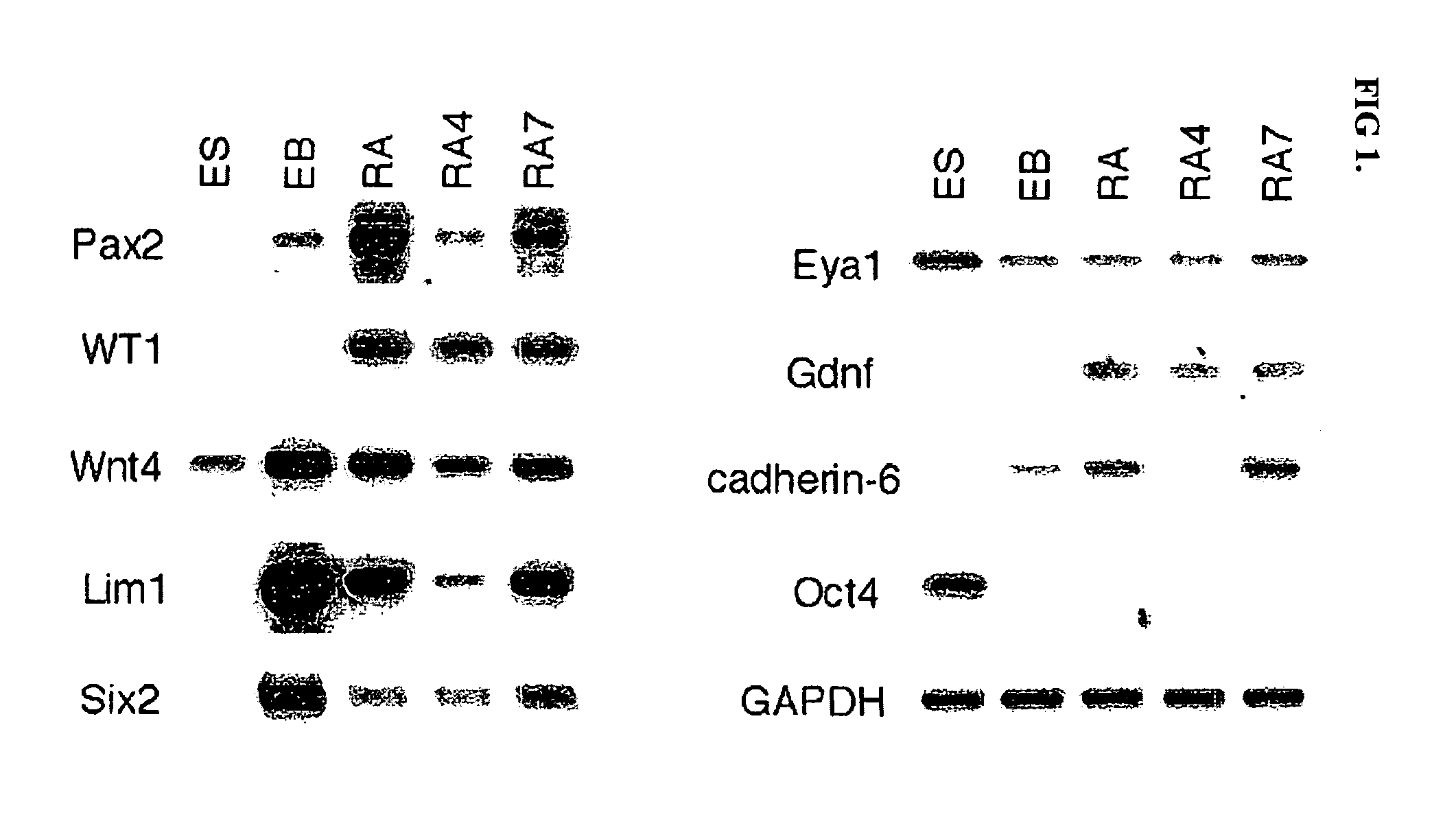
[0073] This example describes cell culture and microinjection techniques utilized in experiments conducted during the course of the present invention. Mouse ES cells (R26) were grown in high glucose DMEM (GIBCO BRL), 10% fetal bovine serum (Atlanta Biologicals), 0.1 mM β-mercaptoethanol (Sigma), 4 mM glutamine (GIBCO BRL), 20 unit / ml PEN / STREP (GIBCO BRL), 0.1 mg / ml G418 (GIBCO BRL), and 103 unit / ml rLIF (Chemicon) on a 0.1% gelatin-coated tissue culture plate at 37° C. CO2 incubator for 2 days. The ES cells were transferred using 0.05% trypsin plus 0.53 mM EDTA (GIBCO BRL) to a 100-mm bacteriological petri dish (Falcon) to induce embryoid body (EB) formation. The EB suspension was cultured in the same medium without rLIF for 2 days and then transferred to 60-mm tissue culture plates coated with 0.1% gelatin. Each 60-mm tissue culture plate contained about 100 EBs. The EBs were grown without growth factors or in the presence of the following growth factors: 0.1 μM retinoic acid (RA)...
example ii
[0074] This example describes a reverse transcription-PCR (RT-PCR) analysis technique utilized in experiments conducted during the course of the present invention. Total RNA was extracted by using TRIZOL reagent (GIBCO BRL). One unit of DNase I (Boehringer Mannheim) was added to 1 μg RNA and the mixture was incubated at 37° C. for 30 min. The isolated RNA by phenol / chloroform method was used for RT-PCR template. Superscript™ One-Step RT-PCR with Platinum Taq (Invitrogen) was utilized for cDNA synthesis and PCR amplification in a Peltier Thermal Cycler (MJ Research). The primer pairs for RT-PCR were as follows:
Pax-2:(SEQ ID NO: 1)CAGCCTTTCCACCCAACG,GTGGCGGTCATAGGCAGCLim-1:(SEQ ID NO: 2)CAAAGAGAACAGCCTCCACTCG,GGATGTGCCAGGATGTCAGTAAATCgdnf:(SEQ ID NO: 3)AAGGTCACCAGATAAACAAGCGG,CATAGCCCAAACCCAAGTCAGTGEya1:(SEQ ID NO: 4)CTAACCAGCCCGCATAGCCG,TAGTTTGTGAGGAAGGGGTAGGSix2:(SEQ ID NO: 5)GCACCTCCACAAGAATGAAAGC,TGAGCAACAGAGCGGGACTGwnt4:(SEQ ID NO: 8)AACTGGAGAAGTGTGGCTGTGACCG,(SEQ ID NO: 7)CATC...
example iii
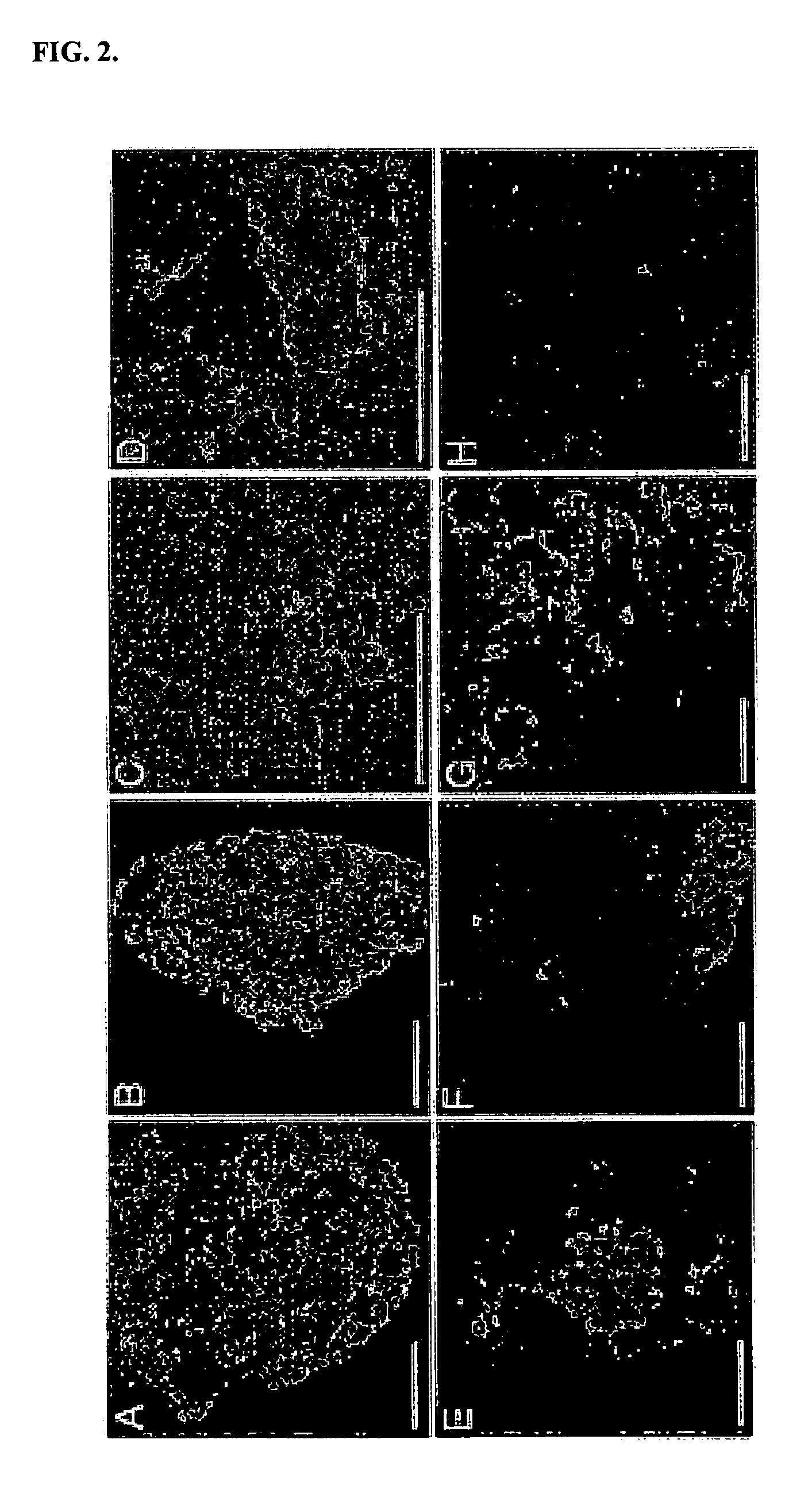
[0075] This example describes immunohistochemistry techniques utilized in experiments conducted during the course of the present invention. Embryoid bodies were fresh frozen in OCT and sectioned at 10 microns in a cryostat. After air drying, sections were fixed in 3% PFA for 10 min and washed in PBS, 0.1% Tween 20 (PBST). Antibodies were incubated for 2 h at room temperature in PBST, 2% goat serum. Primary antibodies used were: rabbit anti-Pax2 (Covance Inc.), mouse anti-pan-cytokeratin (Sigma), mouse anti-E-cadherin (Cell Signaling Technology), mouse anti-b-catenin (Cell Signaling Technology), rabbit anti-laminin (Sigma), and FITC-lotus tetragonobulus agglutinin (LTA, Sigma). After washing two times in PBST, fluorescent conjugated secondary antibodies were used. Images were captured on a Nikon ES800 fluorescent scope with a SPOT digital camera.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com