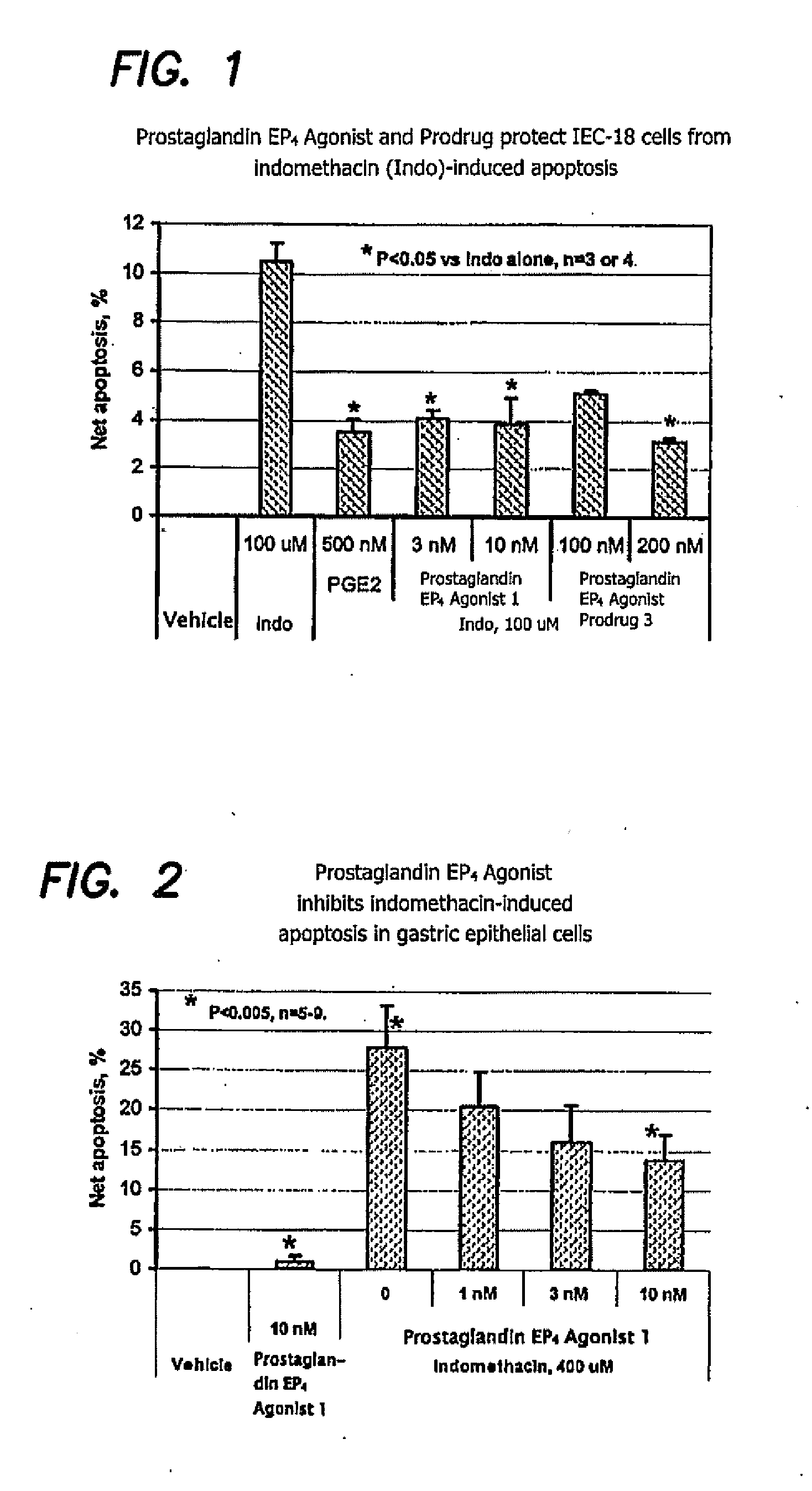
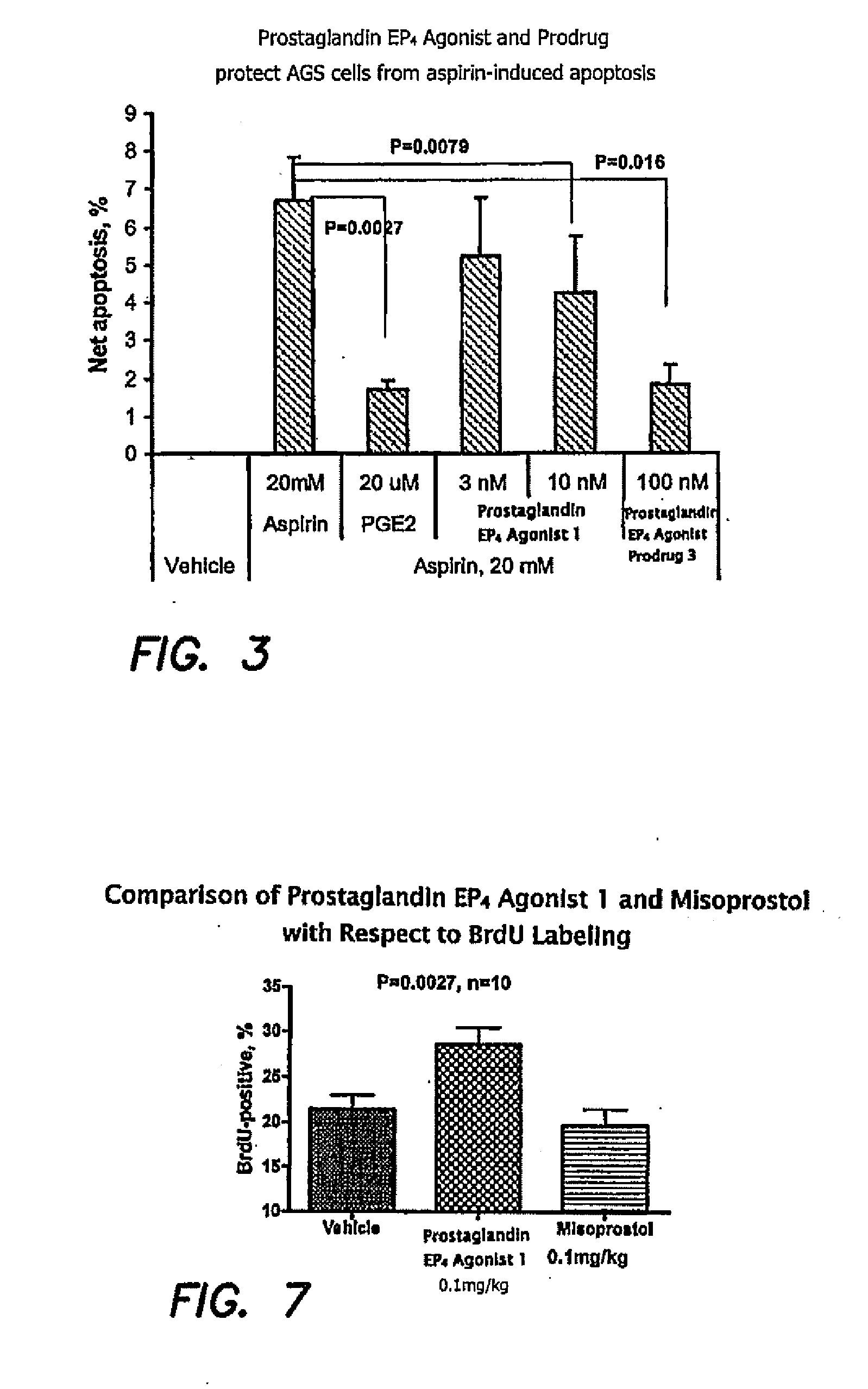
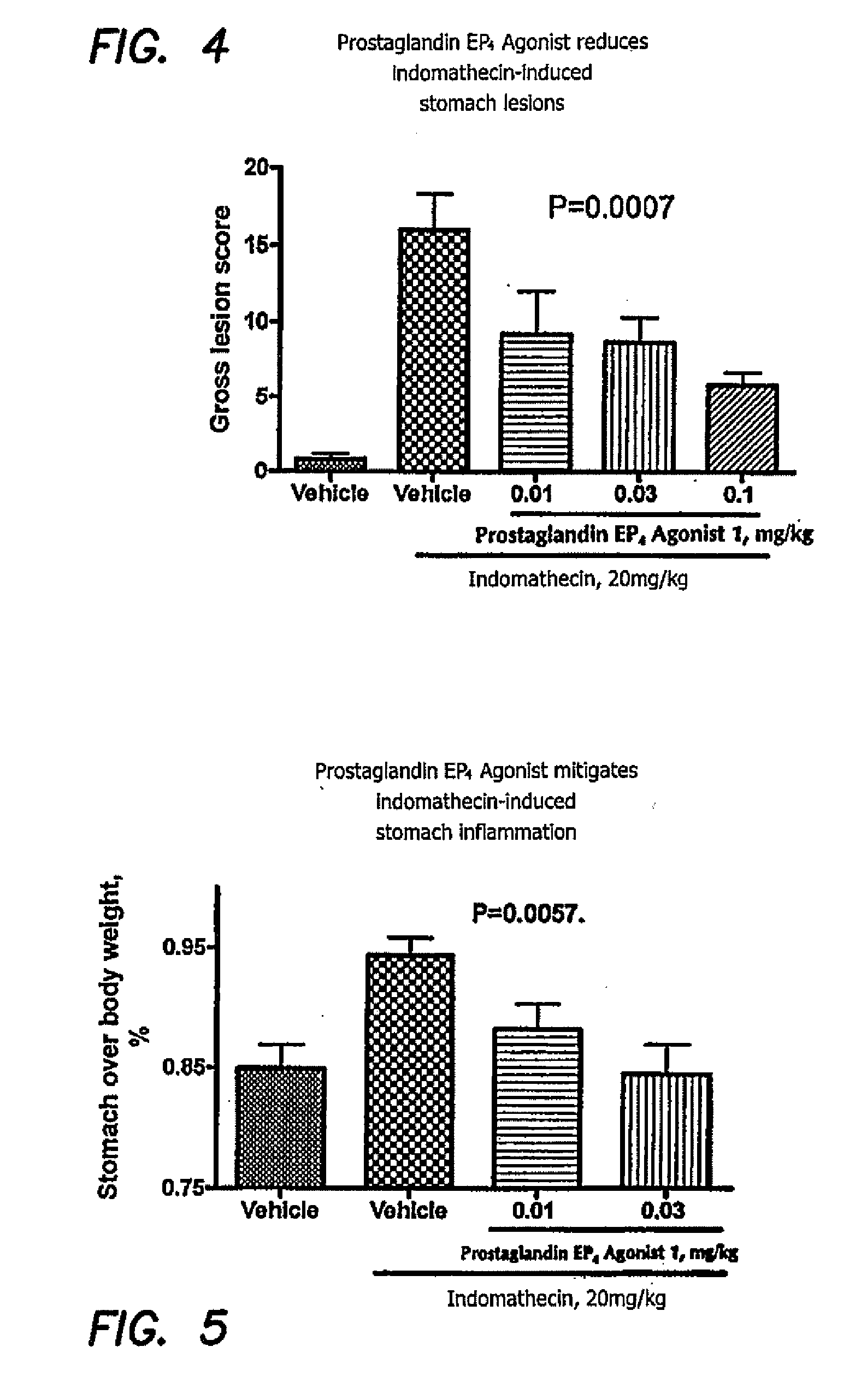
Therapeutic methods using prostaglandin EP4 agonist components
a technology of prostaglandin and components, applied in the direction of biocide, drug composition, elcosanoid active ingredients, etc., can solve the problems of bleeding, erosion, ulcers, etc., and achieve the effect of improving or maintaining the health status of the individual and being easy to practi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 to 5
[0174] A series of five (5) tablet compositions are produced using two (2) different prostaglandin EP4 agonists and three (3) different prostaglandin EP4 agonist prodrugs. Each of the tablet compositions is prepared as follows.
[0175] Within a dust containment area, a mixture of ingredients is prepared and blended until the mixture is uniform. The uniform mixture, having a composition as listed in the table directly below, is then used in a conventional tabletting machine to produce 100 mg tablets having such composition. The tablets may be packaged, for example, in high density polyethylene bottles, with appropriate silica gel packs, capped and labeled.
[0176] The mixtures and tablets have the following make-ups:
Composition12345Ingredientwt. %wt. %wt. %wt. %wt. %Prostaglandin EP410.0————Agonist 1(1)Prostaglandin EP4—10.0———Agonist Prodrug 1(2)Prostaglandin EP4——10.0——Agonist 2(3)Prostaglandin EP4———10.0—Agonist Prodrug 2(4)Prostaglandin EP4————10.0Agonist Prodrug 3(5)Sugar50.050....
examples 6 to 9
[0177] A series of four (4) capsule compositions are produced using two (2) prostaglandin EP4 agonists and two (2) prostaglandin EP4 agonist prodrugs. Each of these capsule compositions is prepared as follows.
[0178] Within a dust containment area, small sugar spheres are provided. An aqueous mixture of the agonist or prodrug including a binder / sealer, such as Opadry® clear, is provided and is sprayed onto the sugar spheres using a conventional fluid bed spraying system. A second mixture including a binder / sealer, e.g., Opadry® clear, in a liquid carrier is sprayed onto the first sprayed spheres using a conventional fluid bed spraying system. This step results in agonist or prodrug loaded pellets with a sealing coat.
[0179] These pellets are coated with an aqueous mixture of triethyl citrate, talc and a methacrylic acid copolymer using a conventional fluid bed spraying system. This step results in agonist or prodrug loaded pellets with a sealing coat and an outer enteric coating. Th...
examples 10 to 14
[0181] Five adult humans are diagnosed with esophageal ulcers. Each of these people orally takes a tablet produced as described in Examples 1 to 5 having a different one of Compositions 1 to 5 once daily for twelve weeks. At the end of this period of time, each of the humans reports substantial relief from the esophageal ulcers. The pain and / or other symptoms of the ulcers have been reduced. In addition the ulcers have been reduced in size or substantially completely healed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com