Integrin-linked kinase and its uses
a technology of integrin-linked kinase and kinase, which is applied in the field of integrin-linked kinase, can solve the problems that to date no cellular protein kinase has been identified that has been demonstrated to bind
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of ILK cDNA
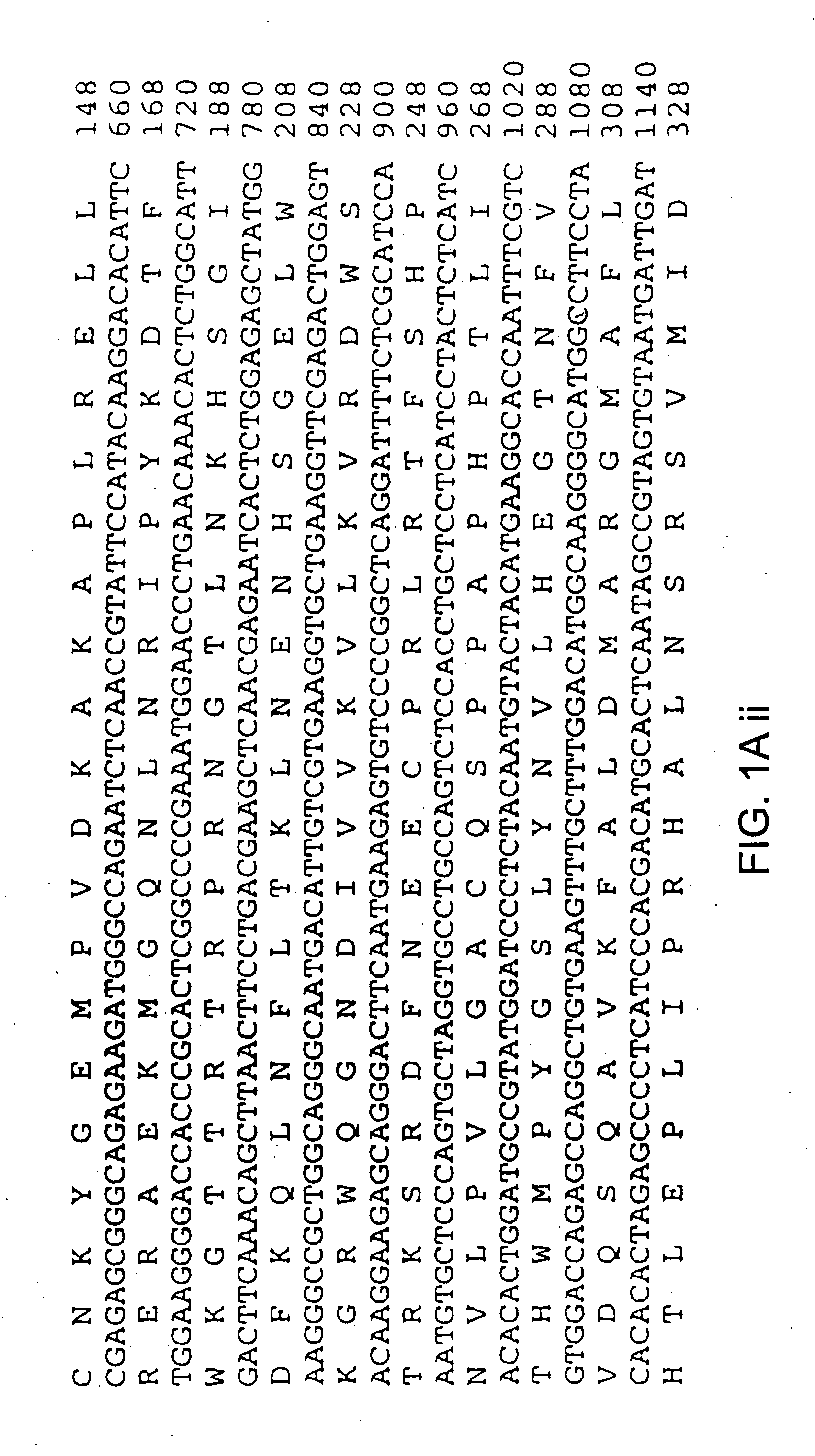
[0107] A partial cDNA, BIT-9, was isolated in a two-hybrid screen using a bait plasmid expressing the cytoplasmic domain of the β1 integrin subunit The BIT-9 insert was used to isolate clones from a human placental cDNA library. A 1.8 kb clone, Plac5, was found to contain a high degree of similarity to cDNAs encoding protein kinases (FIG. 1 a-c), and recognized a widely expressed transcript of 1.8 kb in Northern blots (FIG. 1 d). Deduced amino acid residues 186-451 from Plac5 comprise a domain which is highly homologous with the catalytic domains of a large number of protein tyrosine and serine / threonine kinases (FIG. 1b). Residues 33-164 comprise four repeats of a motif originally identified in erythrocyte ankyrin (FIG. 1 c), likely defining a domain involved in mediating additional protein-protein interactions. Affinity-purified anti-ILK antibodies (see methods described in Example 3) were used in Western blot analyses of mammalian cell extracts, and detected...
example 2
[0110] For analysis of kinase activity in, vitro, a bacterially-expressed fusion protein, GST-ILK132, was SDS-PAGE band puified, and incubated with [γ-32P]ATP in the presence or absence of the exogenous substrate myelin basic protein (FIG. 2). GST-ILK132 autophosphorylated and labeled MBP efficiently in these assays (FIG. 2a). Anti-GST-ILK132 (antibody 91-3) immunoprecipitates of PC3 cell lysates were incubated with [γ-32P]ATP, similar to experiments performed with purified recombinant GST-ILK132. ILK immune complexes labeled a protein of apparent Mr of 59 kDa (FIG. 2b) corresponding to p59ILK, as well as cellular proteins of apparent Mr 32 kDa and 70 kDa, which may be endogenous ILK substrates (FIG. 2b). Cellular phosphoproteins (serine / threonine) of approximately 32 kDa and 70 kDa, were also seen in β1 integrin-specific immune complex kinase assays.
[0111] In ILK immune complex kinase assays a synthetic peptide representing the β3 cytoplasmic domain was phosphorylated, while a sim...
example 3
Association of ILK and β Integrin in Mammalian Cells
[0114] Immunofluorescence experiments indicated that ILK and β integrin co-localize in focal plaques. In order to test further for this association in intact mammalian cells, we performed co-immunoprecipitation assays in lysates of PC3 cells, in which integrin expression has been well-characterized. PC3 cell lysates were immunoprecipitated with specific anti-integrin antibodies, and immune complexes analyzed by Western blotting with the anti-ILK antibody, 92-2. The specificities of the anti-ILK antibodies were tested by immunoprecipitation and Western blotting (FIGS. 3a, b). We detected p59ILK in immune complexes obtained with anti-fibronectin receptor (FNR, α5 / α3 β integrin), and anti-vitronectin receptor (VNR, αvβ3 / β5 integrin) antibodies, but not in those obtained with non-immune serum (FIG. 3c). Three anti-β1 monoclonal antibodies also co-precipitated p59ILK from PC3 lysates, confirming the β integrin specificity of p59ILK int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com