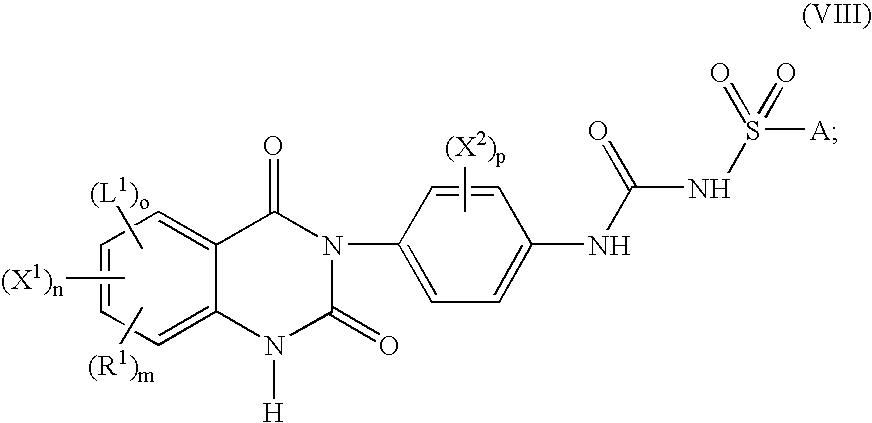
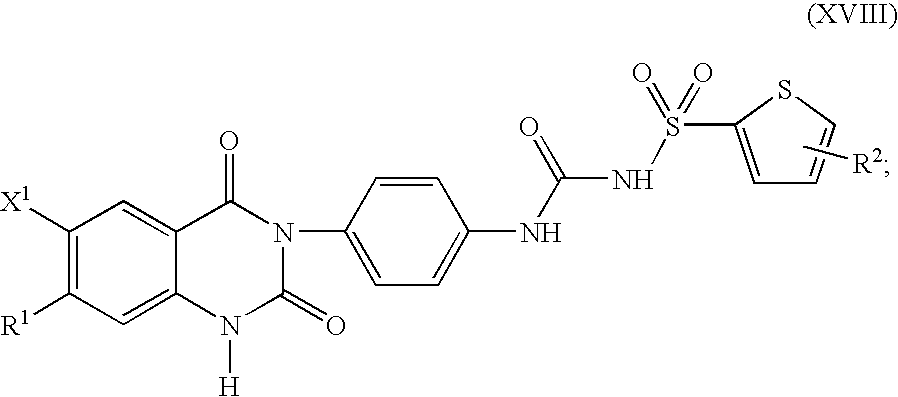
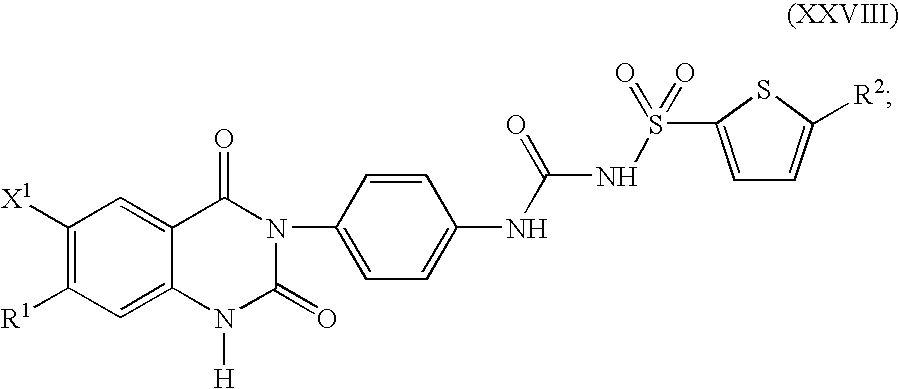
Substituted-(quinazolinyl)phenyl thiophenyl-sulfonylureas, methods for making and intermediates thereof
a technology of phenyl thiophenyl sulfonylurea and substituted(quinazolinyl) phenyl thiophenyl sulfonylurea, which is applied in the directions of drug composition, extracellular fluid disorder, biocide, etc., can solve the problems of thrombotic complications and vascular occlusion, and achieve the effect of prevention or treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of the Intermediate Sulfonylurea Carbamate (8)
[0138]
[0139] The following procedure was adapted from C. A. Hunt, et al. J. Med. Chem. 1994, 37, 240-247. In a three-necked R.B. flask, equipped with a mechanical stirrer, an air condenser, a dropping funnel, and a moisture-guard tube, was placed chlorosulfonic acid (240 mL, 3.594 mol). Under stirring, PCl5 (300 g, 1.44 mol, 0.40 equiv) was added in portions, over ca. 45 mins. During the addition, a large volume of HCl gas evolved vigorously, but the temperature of the mixture did not rise significantly (5 had been added, an almost clear, pale yellow solution resulted, with only a few solid pieces of PCl5 floating in the suspension. It was stirred until gas evolution ceased (0.5 h).
[0140] Then the reaction vessel was cooled in ice, and 2-chloro-thiophene (66.0 mL, 0.715 mol) was added via the dropping funnel, over 1.0 h. With the addition of the very first few drops of 2-Cl-thiophene, the mixture turned dark purple, and by th...
example 2
Synthesis of [4-(6-fluoro-7-methylamino-2,4-dioxo-1,4-dihydro-2H-quinazolin-3-yl)-phenyl]-5-chloro-thiophen-2-yl-sulfonylurea (7a)
[0148]
Step 1
[0149] Aniline 1 (1H NMR (DMSO): δ 7.58 (dd, 1H), 6.72 (dd, 1H), 3.77 (s, 3H); 6.0 g, 32.085 mmol) was placed in a 500 mL round bottomed flask and 20% phosgene in toluene (175 mL, 332.50 mmol, 10.36 equiv) was added. The resulting somewhat sticky suspension was then magnetically stirred overnight at room temperature resulting in a clear, colorless solution. An aliquot removed, blown dry with argon, quenched with MeOH, and analyzed by RP-HPLC / MS to show no unreacted aniline 1 and clean formation of the isocyanate 2a and / or carbamoyl chloride 2b as analyzed as its methyl-carbamate. The mixture was concentrated first by rotary evaporation and then under high vacuum to yield 6.76 g (99% yield) of the isocyanate 2a and / or carbamoyl chloride 2b as a free-flowing colorless solid.
Step 2
[0150] In a 500 mL R. B. flask was placed N-Boc-1,4-phenyle...
example 3
Synthesis of [4-(6-fluoro-7-methylamino-2,4-dioxo-1,4-dihydro-2H-quinazolin-3-yl)-phenyl]-5-chloro-thiophen-2-yl-sulfonylurea (7a)
[0157]
Step 1:
[0158] Methyl 2-amino-4,5-difluorobenzoate [2] (38 Kg, 1.0 eq) and dichloromethane (560 Kg, 8×, ACS>99.5%) were charged to a PP1-R1000 reactor (2000L GL reactor). The reaction mixture was agitated for 5 mins. 4-Nitrophenylchloroformate (49.1 Kg, 1.2 equiv) was charged into PP1-R2000 reactor (200L) followed by dichloromethane (185 Kg) and agitated the contents for 5 mins. After pressurizing the 200L reactor the 4-nitrophenylchloroformate solution was transferred into the 2000L reactor containing dichloromethane solution of [2]. The reaction mixture was heated to 40±5° C. (reflux) under nitrogen gas purge for 3 hrs. The representative TLC analysis confirmed reaction completion (in-process TLC, no compound 2 remaining; 99:1 CHCl3—MeOH). The solution was cooled to 30° C. and distilled off 460 Kg of dichloromethane under vacuum. The 2000L reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com