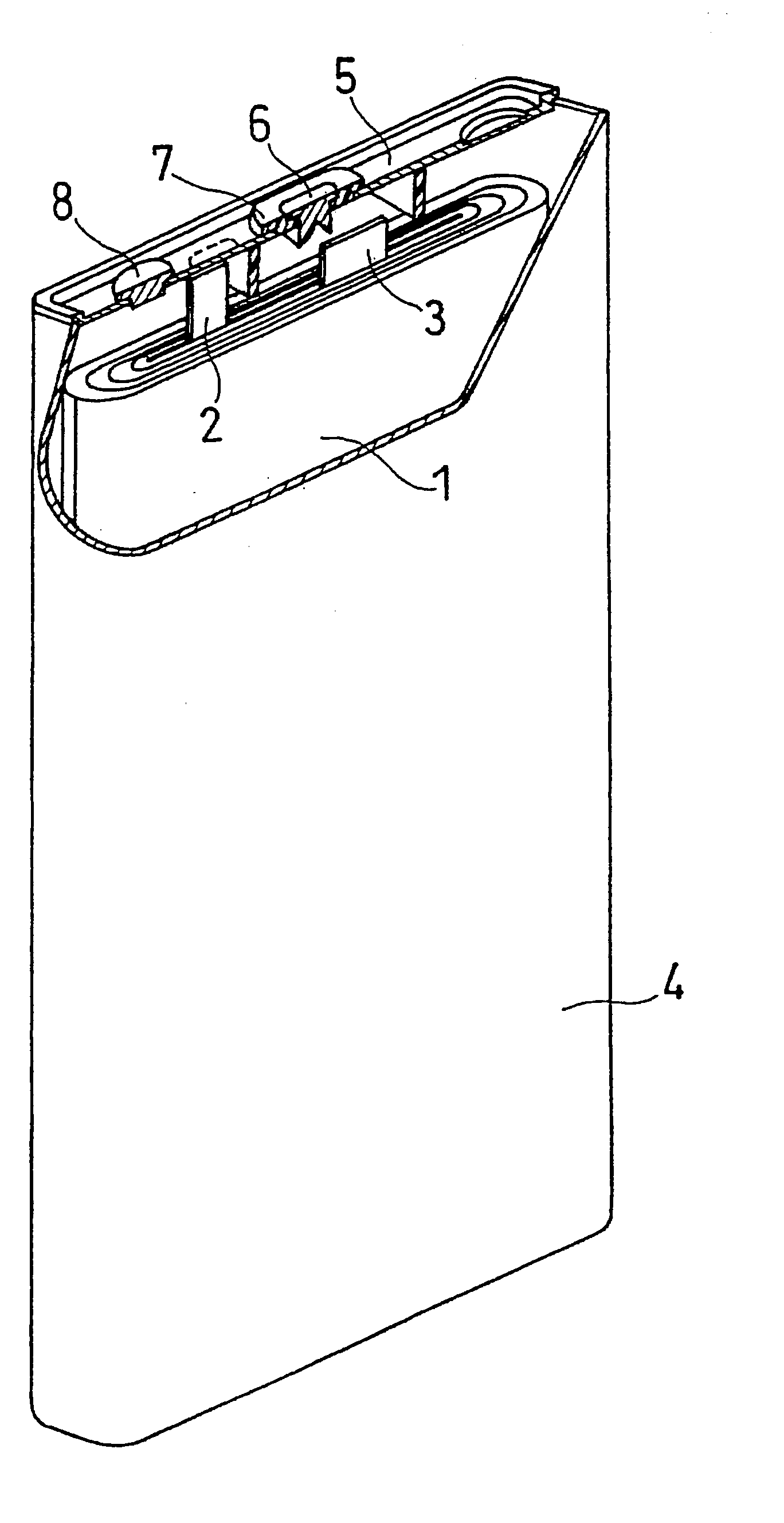
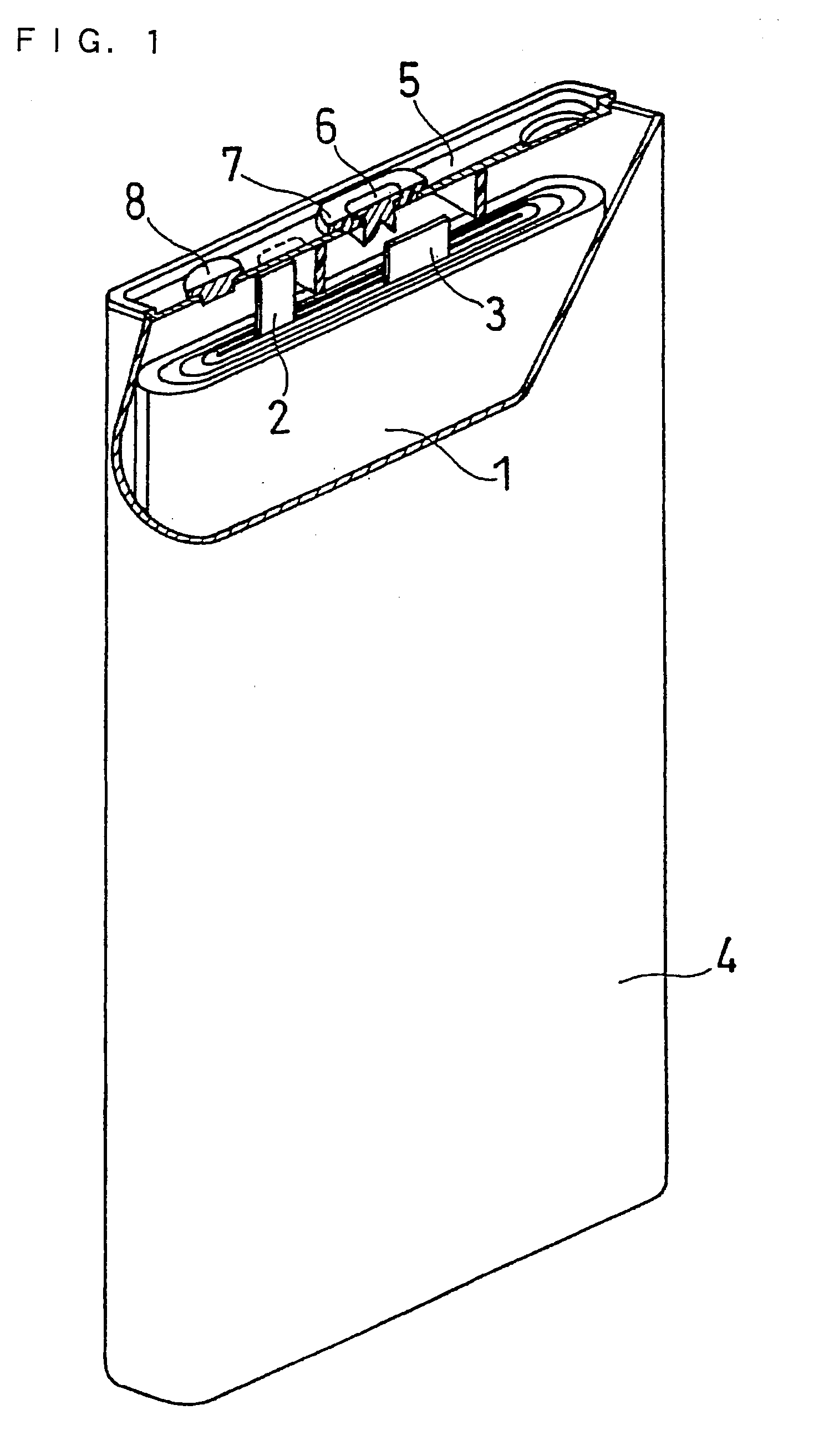
Non-Aqueous Electrolyte and Secondary Battery Containing the Same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0087] (i) Fabrication of a Positive Electrode Plate
[0088] LiCoO2 (mean particle size 10 μm) serving as a positive electrode active material, carbon black serving as a conductive agent, and polyvinylidene fluoride (PVdF) serving as a binder were blended at a mass ratio of 100:3:4, then kneaded with an appropriate amount of N-methyl-2-pyrrolidone (NMP) to give a positive electrode material mixture paste.
[0089] The positive electrode material mixture paste was applied on both faces of a positive electrode current collector formed of an aluminum foil having a thickness of 30 μm by means of a doctor blade method so that a thickness after drying became approximately 230 μm. Then the current collector with paste was pressed so that a dry coating membrane had a thickness of 180 μm, and was cut into a predetermined size to obtain a positive electrode plate. To the positive electrode plate, a positive electrode lead made of aluminum was welded.
[0090] (ii) Fabrication of a Negative Electro...
example 2
[0108] A rectangular lithium ion secondary battery was fabricated in the same manner as in Example 1 except that the content of the hydrogenated m-terphenyl in the non-aqueous electrolyte was changed to 0.2 mass %.
example 3
[0109] A rectangular lithium ion secondary battery was fabricated in the same manner as in Example 1 except that the content of the hydrogenated m-terphenyl in the non-aqueous electrolyte was changed to 0.5 mass %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com