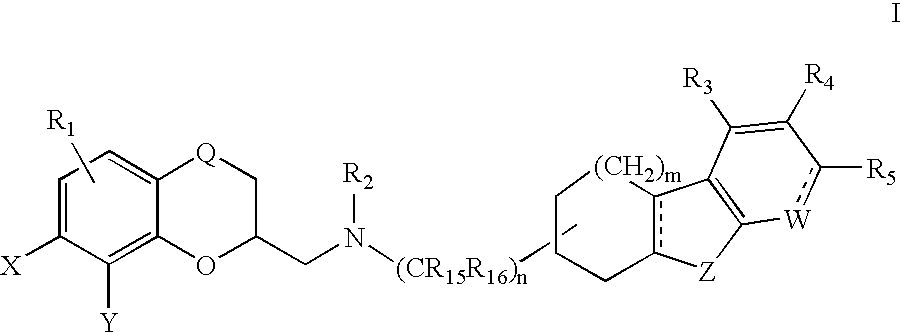
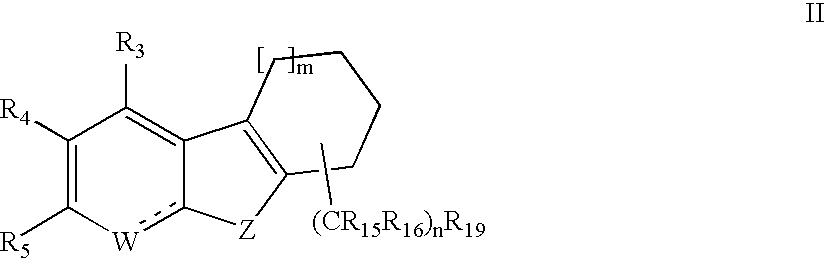
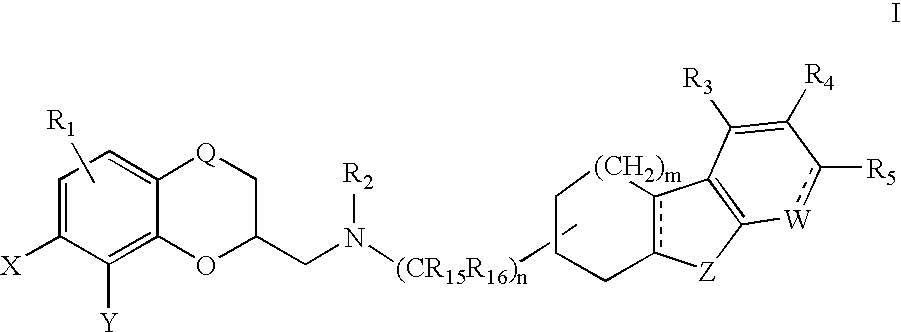
Cycloalkylfused indole, benzothiophene, benzofuran and indene derivatives
a technology of cycloalkylfused indole and indene derivatives, which is applied in the direction of drug compositions, sexual disorders, metabolic disorders, etc., can solve the problems of reducing the latency period, reducing the energy or motivation of people, and feeling of sadness or emptiness, so as to increase the serotonin level and reduce the latency period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-[(6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-2-yl)methyl]-N-{[(2S)-8-methyl-2,3-dihydro[1,4]dioxino[2,3-f]quinolin-2-yl]methyl}amine
6-Fluoro-2,3,4,9-tetrahydro-1H-carbazole-2-carboxylic acid (I)
[0248] 4-fluorophenylhydrazine (0.90 g, 7.14 mmol) was dissolved in glacial acetic acid (25 mL) under a nitrogen atmosphere and added dropwise to a refluxing solution of 3-cyclohexanonecarboxylic acid ethyl ester (1.73 g, 10 mmol) dissolved in glacial acetic acid (15 mL). After the addition was complete, the solution was heated under reflux for 1 hr, cooled to room temperature and allowed to stir overnight. The orange solution was evaporated under reduced pressure to give a yellow-brown solid. The solid was suspended in 10% sulfuric acid and heated under reflux for 5 hrs, cooled to room temperature and allowed to stir overnight. The brown solid was collected by filtration and recrystallized from acetic acid and water to give the compound I (1.21 g), mp: >200° C. MS [M−H] m / z 232.
[(2S)-8-met...
example 2
N-[(6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-yl)methyl]-N-{[(2S)-8-methyl-2,3-dihydro[1,4]dioxino[2,3-f]quinolin-2-yl]methyl}amine
[0253] 6-Fluoro-2,3,4,9-tetrahydro-1H-carbazole-3-carboxylic acid ethyl ester (III)
[0254] 4-Cyclohexanonecarboxylic acid ethyl ester (25 g, 0.14 m) and 4-fluorophenyhydrazine hydrochloride (22.5 g, 0.13 m) were dissolved in ethanol (450 mL) and heated under reflux for 16 hrs. After cooling, the white solid was filtered off and the solvent removed under reduced pressure. After partitioning the residue between water and ethyl acetate, the organic portion was separated, dried (MgSO4), and evaporated under reduced pressure to give compound III (35.5 g, 0.13 m). The crude product was recrystallized from heptane. mp: 115-117° C. MS: [M+H]+@m / e=262. [Lit. ref: Block, M. H., et al. J. Med. Chem. 2002,45, 3509].
(6-Fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-yl)-methanol (IV)
[0255] Lithium aluminum hydride (800 mg) was added portion-wise to a solution of ester III...
example 3
S,S-(6-Fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-ylmethyl)-(8-methyl-2,3-dihydro-[1,4]dioxino[2,3-f]quinolin-2-ylmethyl)-amine
[0258] The mixture of diastereomers in Example 2 was separated by chromatography using a Varian Prep with Chiralpak AD (0.2×15 cm); mobile phase: methanol with diethylamine. The title product [mp 80-84° C.; MS (ES) m / z 430.2] was dissolved in ethanol and treated with two equivalents of ethereal HCl. Yellow dihydrochloride precipitated out immediately. The compound was isolated by filtration and was washed with ethanol. After drying in vacuo, the title compound was obtained as the dihydrochloride sesquihydrate. mp 253-263° C., MS: [M+H]+ @ (m / z 432.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com