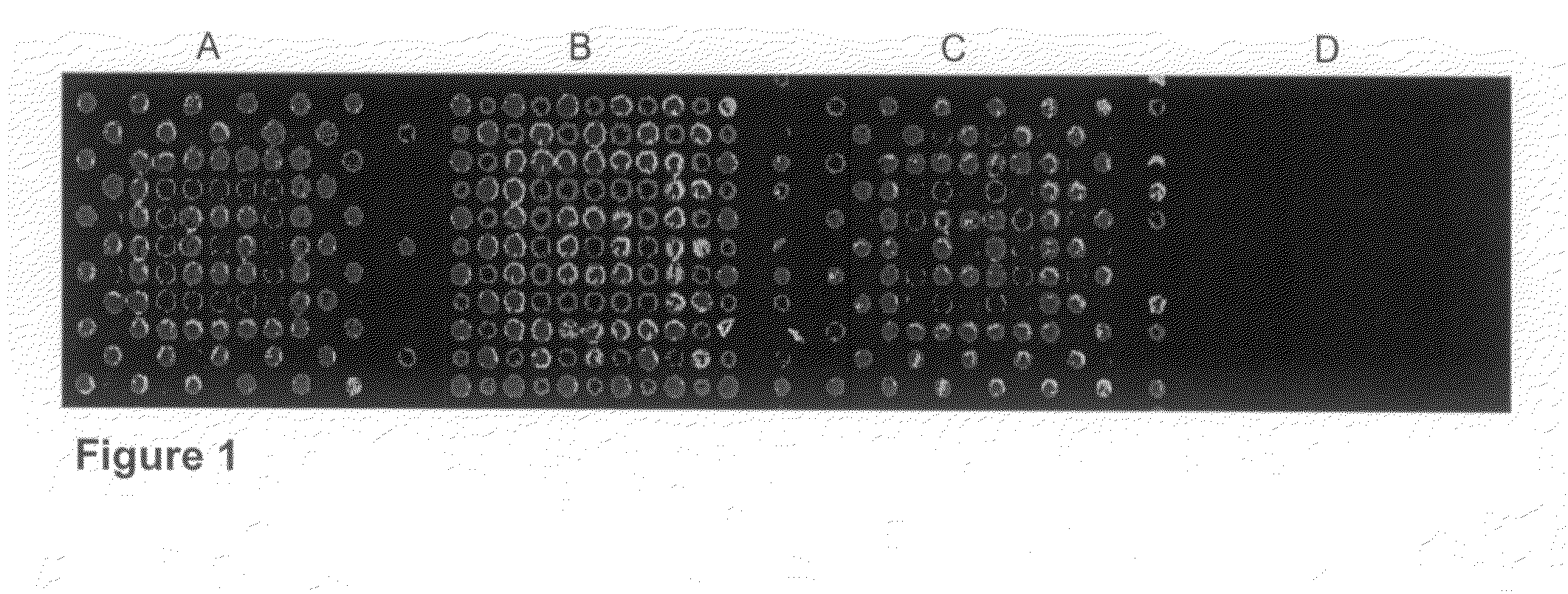RNA sequences generated using a microarray having a base cleavable succinate linker
a technology of succinate linker and rna sequence, which is applied in the field of double stranded rna compounds, can solve the problems of low yield, difficult to access target sequences in the mrna, and reaction conditions that require a relatively long time period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
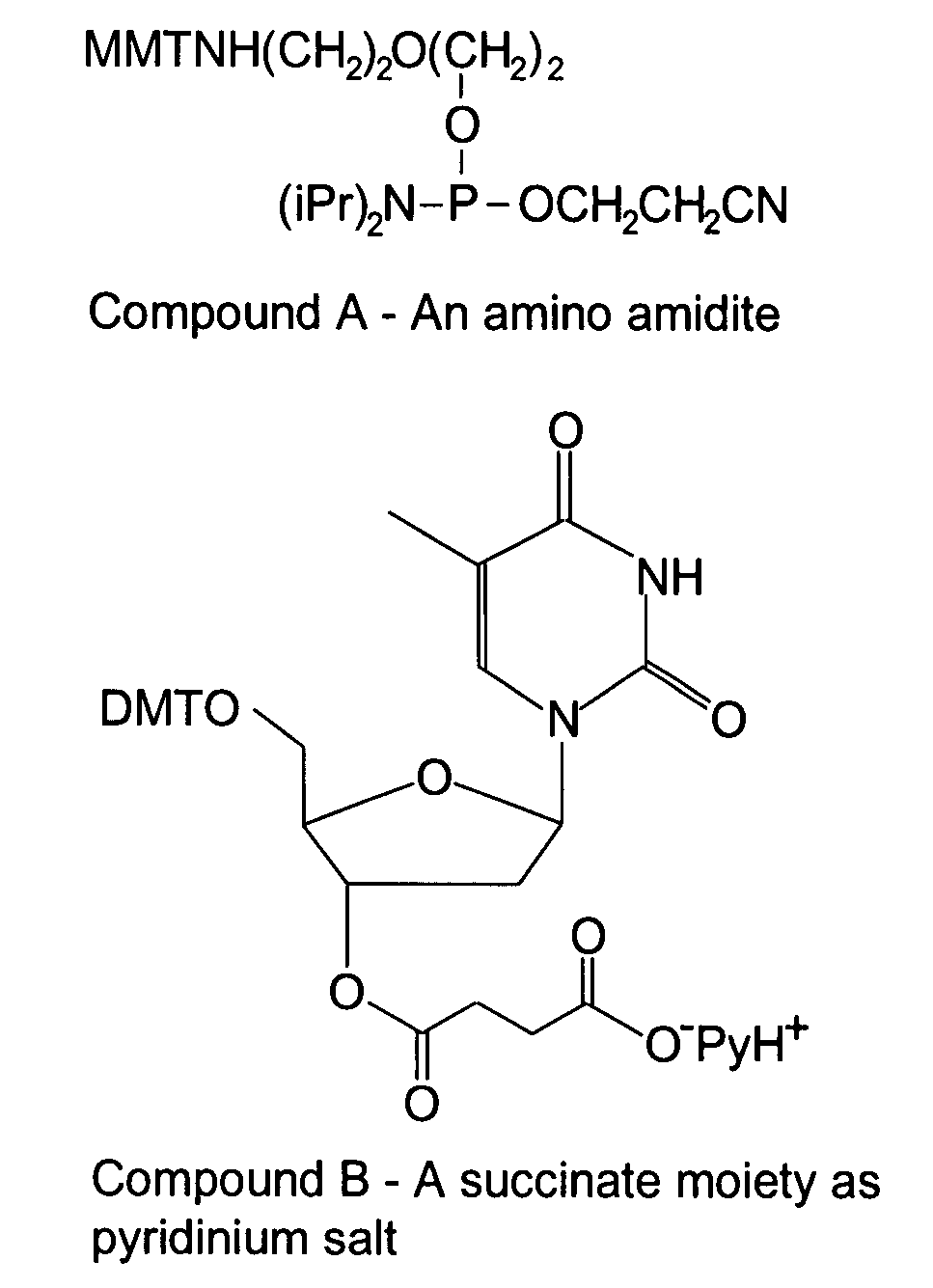
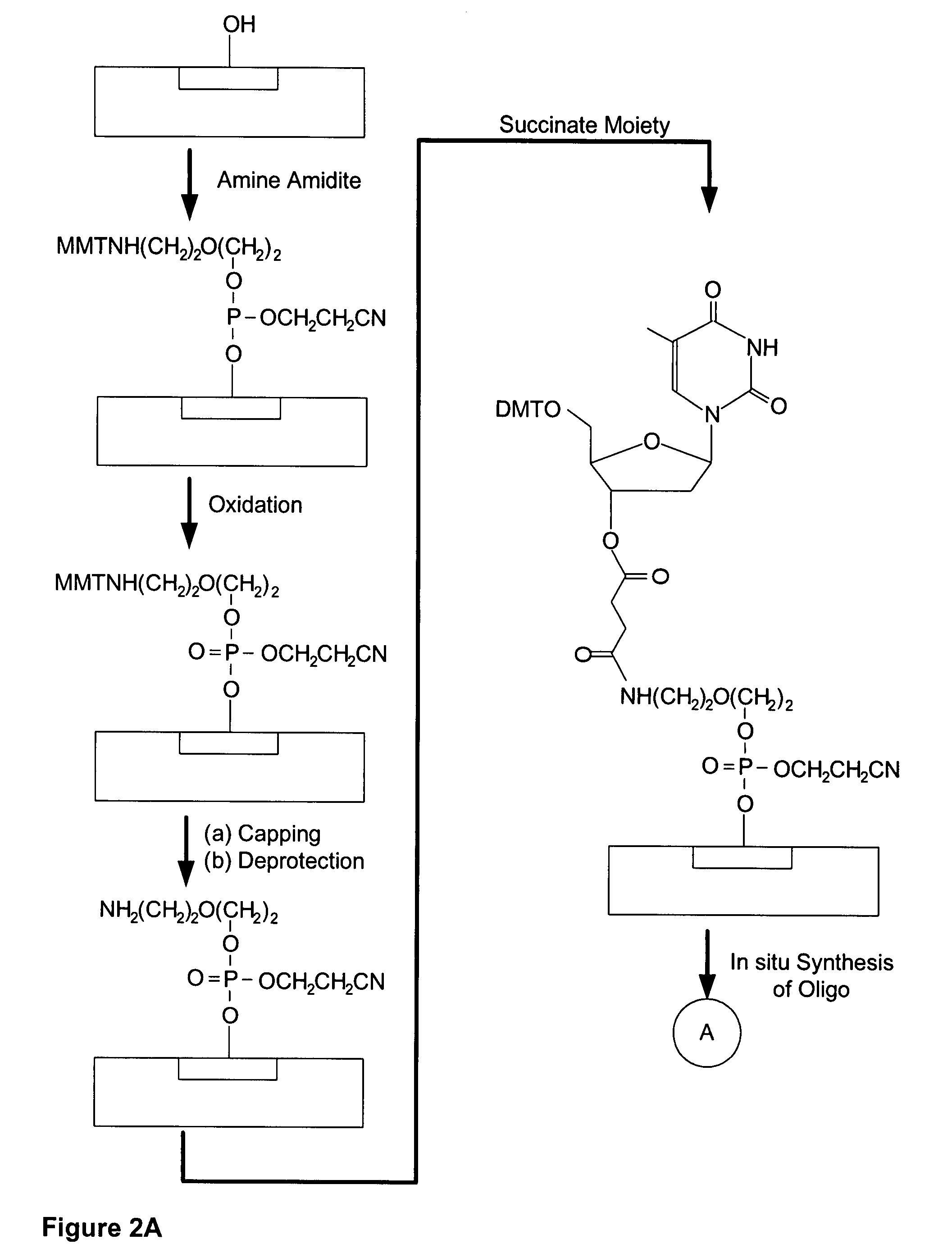
Cleavable Linker Using Amino Amidite and T-Succinate
[0153]In this example, a CombiMatrix CustomArray™ 12K microarray was used to synthesize oligonucleotides attached to the microarray through a base-cleavable linker. The microarray had approximately 12,000 platinum electrodes on a solid surface having a porous reaction layer, wherein each electrode was electronically addressable via computer control. The oligonucleotides were DNA and were synthesized in situ using electrochemical synthesis at locations associated with the electrodes on the microarray. Electrochemical synthesis used standard phosphoramidite chemistry coupled with electrochemical deblocking of the protecting groups on the synthesized DNA for the addition of each nucleotide contained in the oligonucleotide. For attachment of the phosphoramidites, the microarray had organic reactive hydroxyl groups that allowed attachment of the first phosphoramidite. Electrochemical deblocking involved turning on an electrode to genera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| electric field | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com