Composition for treating a disease caused by neuronal insult comprising a human umbilical cord blood-derived mesenchymal stem cell as an active ingredient
a technology of umbilical cord blood and stem cells, applied in the direction of biocide, drug composition, nervous disorder, etc., can solve the problems of cell engraftment and survival ability degrade, secondary injury, and difficulty in implementing cell engraftment or cell survival, so as to confirm the therapeutic effect of nerve damage-related diseases, the effect of improving the recovery of behavior motor ability and facilitating the migration of transplanted cells to the lesion area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
First Embodiment
Experiment using a Spinal Cord Injury Model of White Rat
[0060]1. Material and Method
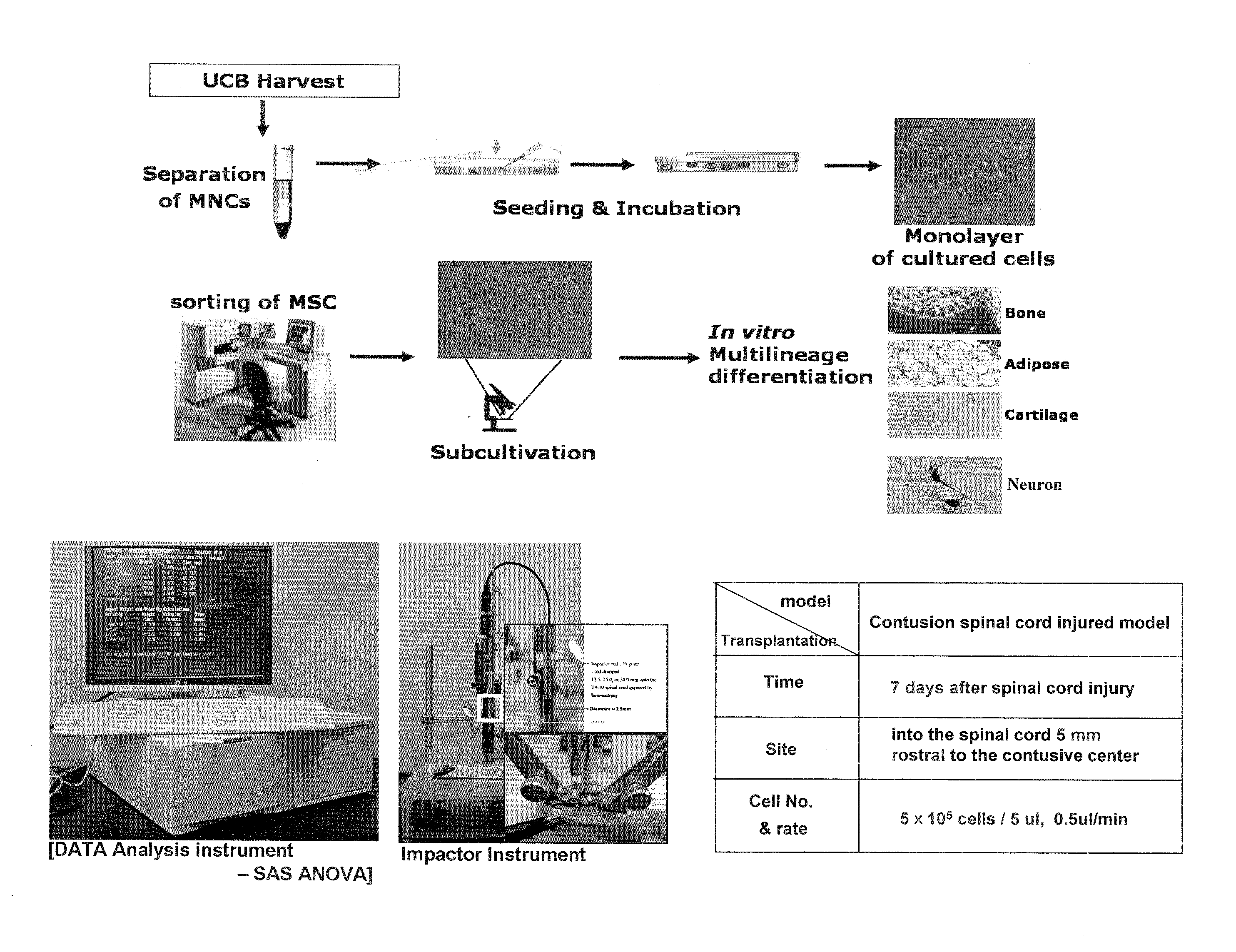
[0061]1.1. Separation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell
[0062]Human umbilical cord blood obtained with parental consent were separated by Ficol gradient (density 1.077 g / cm3, Sigma Co.) to clean mononuclear cells, which were then suspended by α-MEM (Gibco BRL) contained with 10% FBS (HyClone) and divided at a concentration of 5×106 cells / cm2. An incubating solution was exchanged two times a week, and the cells were incubated with 5% CO2 at 37° C.
[0063]When incubation of mononuclear cells derived from the human umbilical cord blood was established and fibro blast like adhesive cells were observed, 0.25% Trypsin (HyClone) was processed to separate cells and then was suspended again by an incubating solution when a monolayer of colonies was 80% after three weeks.
[0064]After the cells were secondarily incubated with a dimension of 5×104 cells / cm2, they were in vit...
second embodiment
Effect of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell on Endogenous Neurogenesis of Spinal Cord Injury of Contused Mature White Rat
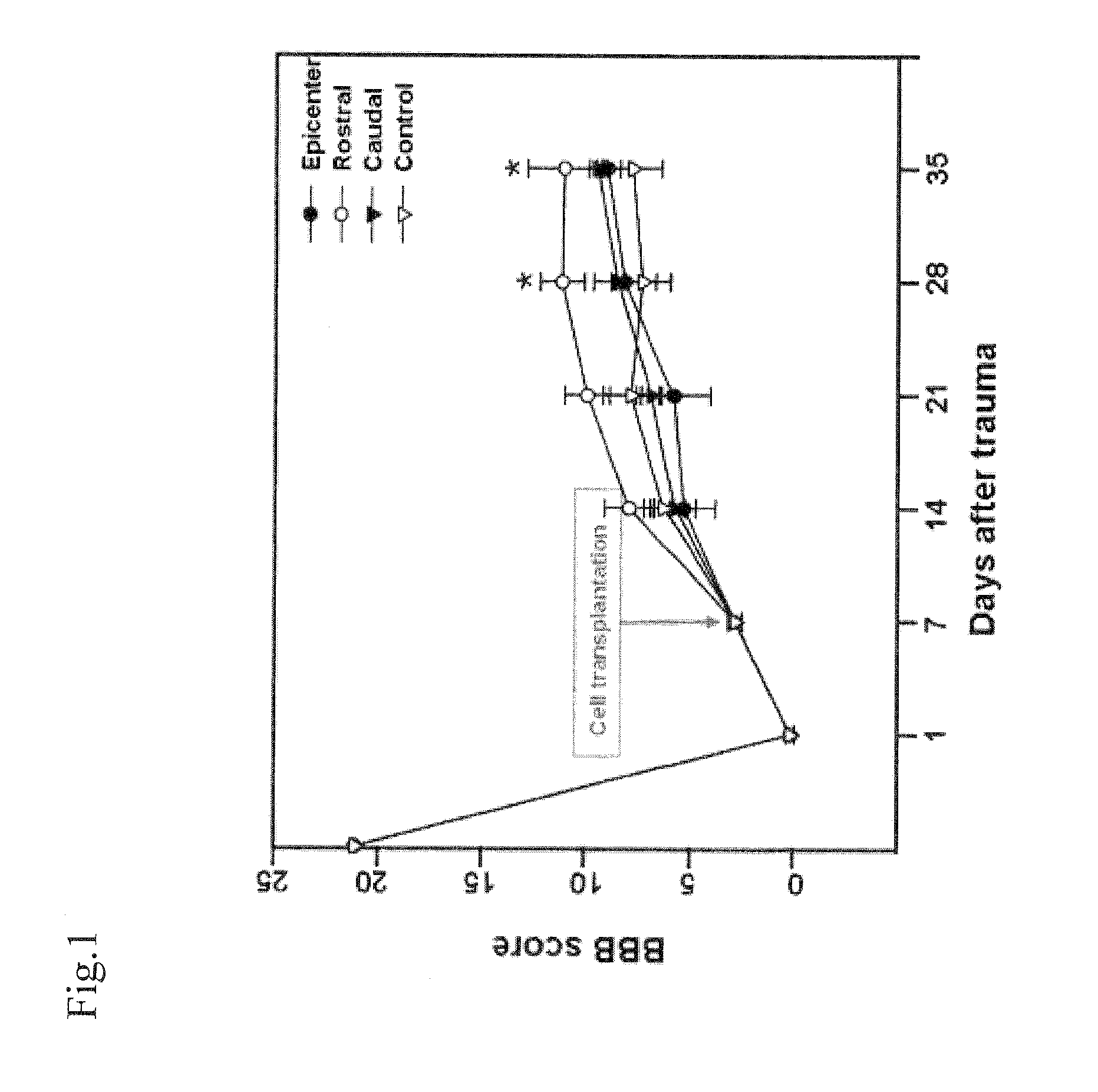
[0096]In the present embodiment, it was checked 1) whether the transplanted hUCB-derived MSCs can improve a function outcome of the mature rat having the contusive spinal cord injury (SCI), 2) whether the hUCB-derived MSCs can be differentiated and expressed into a nerve or neuroglial cell and 3) whether the hUCB-derived MSCs derive the expression of the neurotrophic facto regenerated after SCI or proliferation of intrinsic endogenous neural stem / progenitor cells.
[0097]After the human umbilical cord blood-derived mesenchymal stem cell was separated in accordance with the method of FIG. 5 and transplanted, intraperitoneally BrdUrd (50 mg / kg, Sigma-Aldrich) was injected into the SD rat for labeling a newly generated cell for 14 days. Then, the checked proliferation reaction is focused on cytogenetics in which cells are generated within 14 days...
third embodiment
Effect of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell on Treatment of Stroke in a White Rat
[0110]In the present embodiment, a functional test, migration to the injured area, and differentiation into a nerve cell after UCB-MSCs transplantation will be described.
[0111]Focal cerebral ischemia was derived by intraluminal thread occlusion of middle cerebral artery (MCA). At the seventh day of transplantation, the PKH-26 labeled UCB-MSCs (3×105 cells / 5 μl) were transplanted to the injured cerebrum contralateral lesion of the rat.[0112]Focal cerebral ischemia model
[0113]Sprague-Dawley male (rat weight): 230-250 g
[0114]An intraluminal filament technique (Middle cerebral artery occlusion; MCAO) was performed on transient focal cerebral ischemia.
[0115]4-0 monofilament suture; round end
[0116]120 minutes MCA occlusion; a heating pad was used to remain at 37° C.[0117]MCAO rotarod test
[0118]Adhesive-removal test: ¢ 12, 113.1 mm2 paper dot
[0119]Rotarod test; 4-40 rpm, accelerated test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com