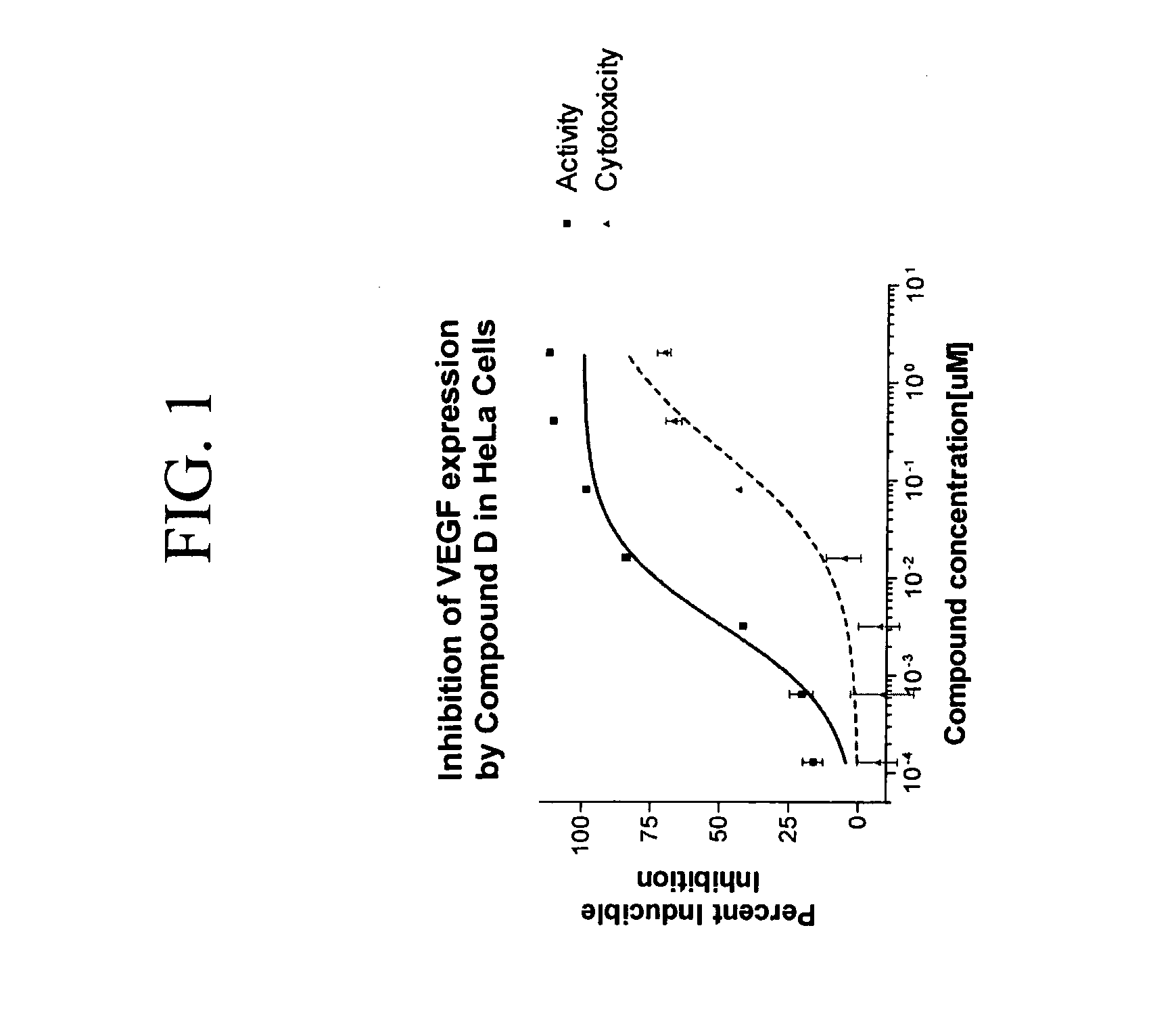
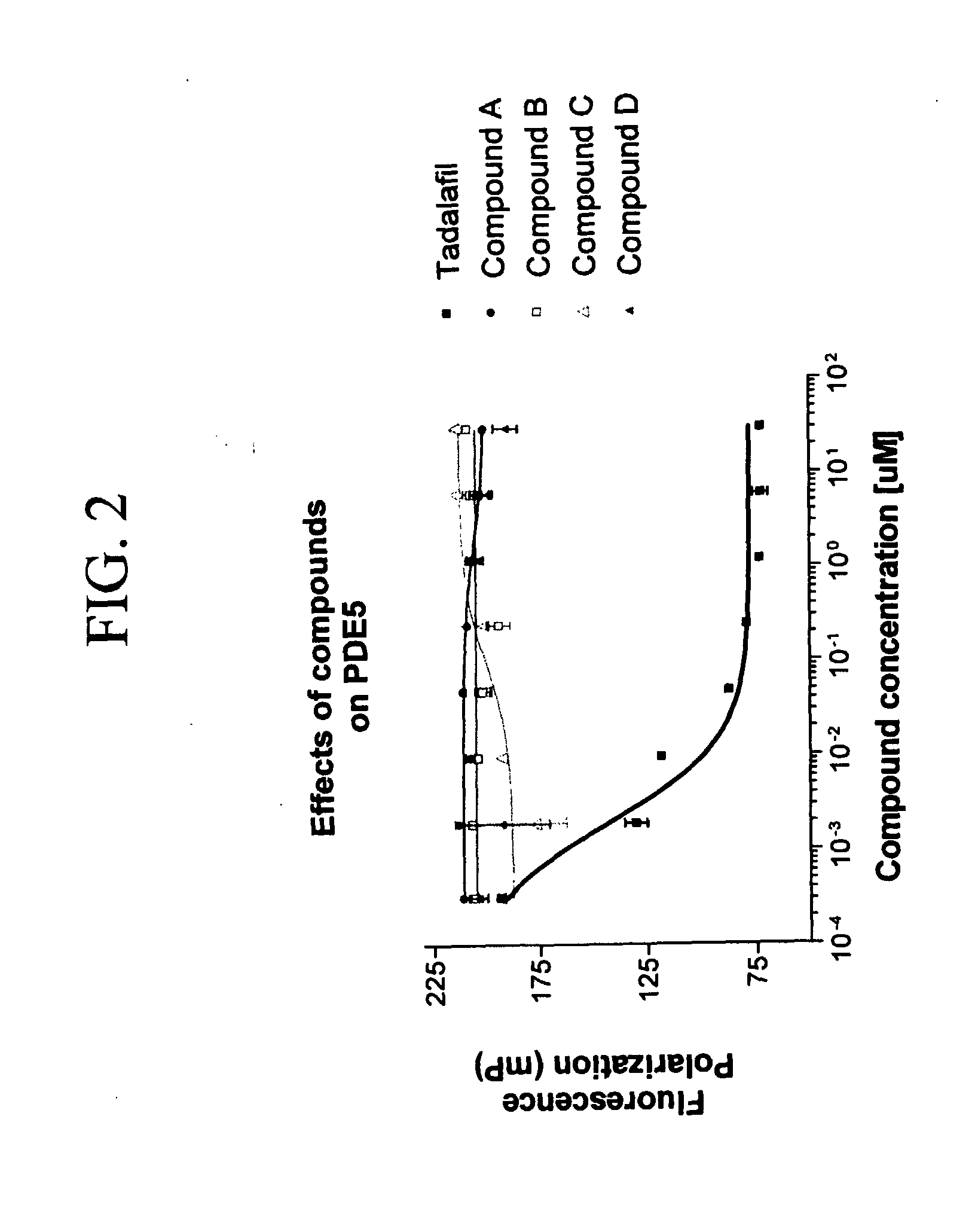
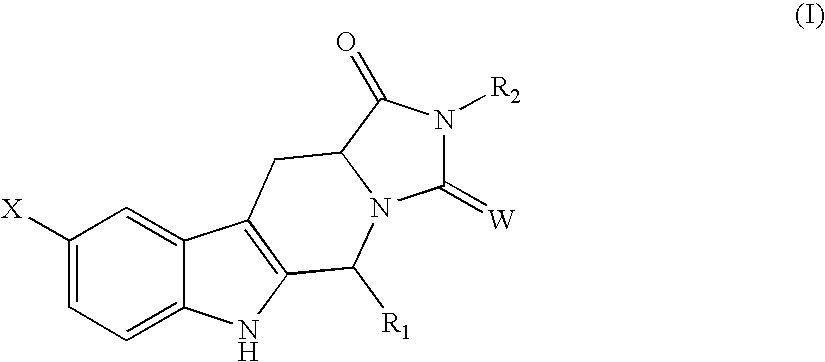
Tetra-Cyclic Carboline Derivatives Useful in the Inhibition of Angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compounds of the Invention
[0112]Compounds of Formula I may be prepared according to Scheme I. By way of example, Compounds 23, 33, 35 and 40 may be prepared as follows. Other preferred compounds of the invention, such as those in Table 4 below, may be similarly prepared.
example 1a
Synthesis of Compound 2 (Racemic)
[0113]
[0114]In accordance with Scheme I, p-anisaldehyde (2.16 g, 15.9 mmol, 1.93 mL) is added to a suspension of 5-Bromotryptophan (3 g, 10.6 mmol) in 100 mL of Acetic acid at room temperature. The reaction mixture is then heated to reflux at about 125° C. in silicon oil bath and maintained at that temperature for about 3 hours 20 minutes. The resulting solution is concentrated under vacuum. The residue is triturated with dichloromethane, diethyl ether and hexane to yield powdery brown solid. The resulting acid salts of the desired product are then collected and washed with hexane three times and then, used for next step without purification.
[0115]The collected brown solid is suspended in 60 ml of acetone. The suspension is treated with triethylamine (14.37 mmol, 2 mL) and cyclohexyl isothiocyanate (2.03 g, 14.37 mmol, 2.04 mL) to give a homogenous solution. The reaction mixture is refluxed for about 2.5 hours at about 70° C., and then concentrated u...
example 1b
Synthesis of Compound 14
[0116]
[0117]In accordance with Scheme I, p-anisaldehyde (0.18 ml, 1.5 mmol) is added to 5-bromotryptophan (283 mg, 1.0 mmol) in AcOH (1.5 ml) and heated to about 110° C. in a capped tube. The solids are dissolved upon heating. After about 2.5 h, the reaction mixture is cooled to room temperature and concentrated on rotavap. The thick oil residue is stirred in CH3CN (2 ml) and a brownish solid precipitated out. The solid is filtered and washed with CH3CN (2×) to give 5 as a light brown powder, 0.20 g, 50% yield. The product was 95% pure by LC-MS, contaminated with 3% of 4 and 2% of p-anisaldehyde, and it is used without further purification. MS (ES+) m / z: 401 / 403.13, Rt: 2.45 min.
[0118]To a solution of 5 (55.5 mg, 1.5 mmol) in 2-butanone (1.5 ml) and TEA (0.2 ml) is added cyclohexylisocyanate. The mixture is heated at 100° C. in a capped tube. After 2 h, the reaction mixture was cooled to room temperature, concentrated on rotavap. The residue was chromatograph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com