Screening for gestational disorders
a gestational disorder and screening technology, applied in the direction of instruments, material analysis, biological material analysis, etc., can solve the problems of increased risk of pih, significant maternal and fetal morbidity and mortality, and increased cesarean section ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
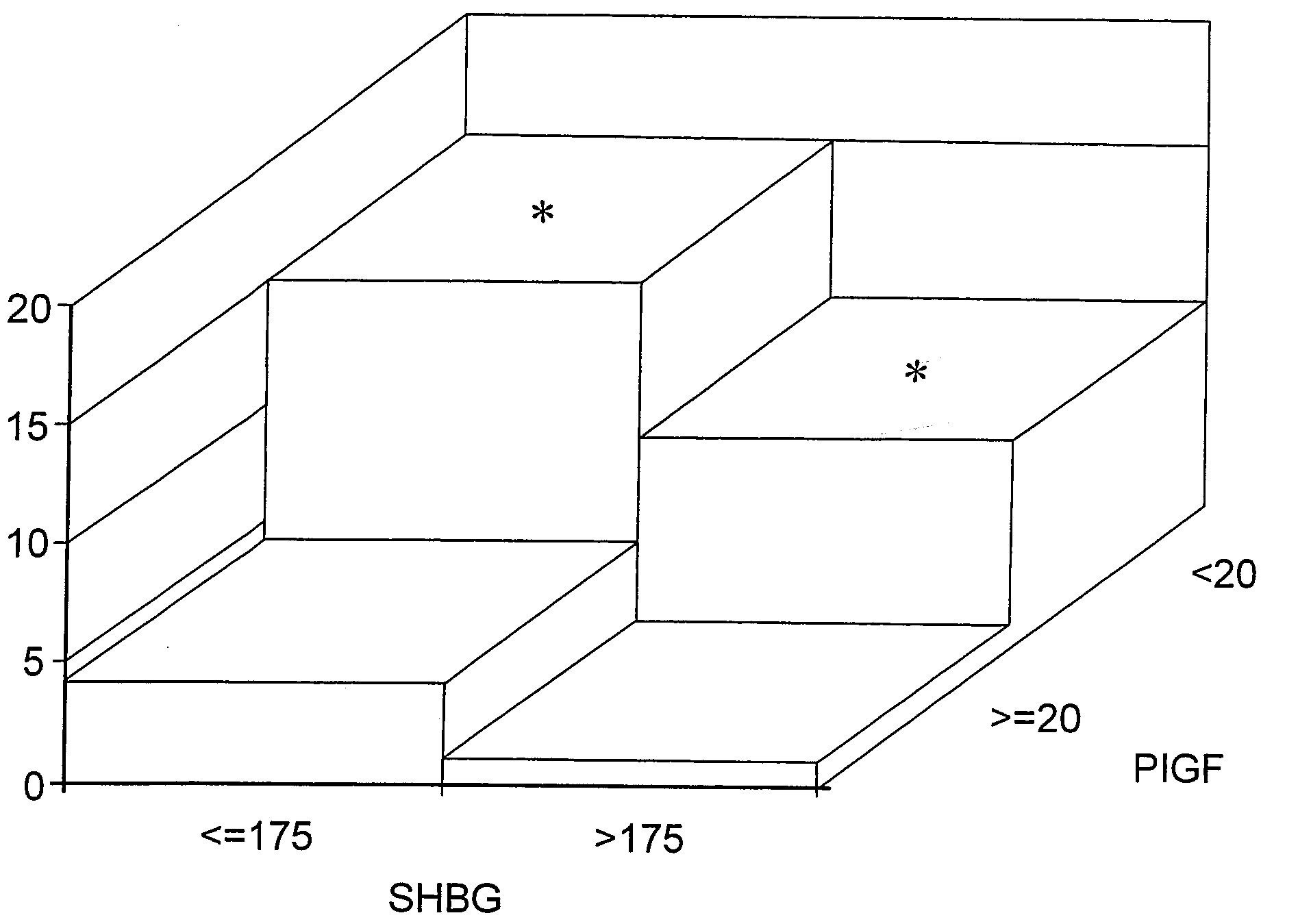
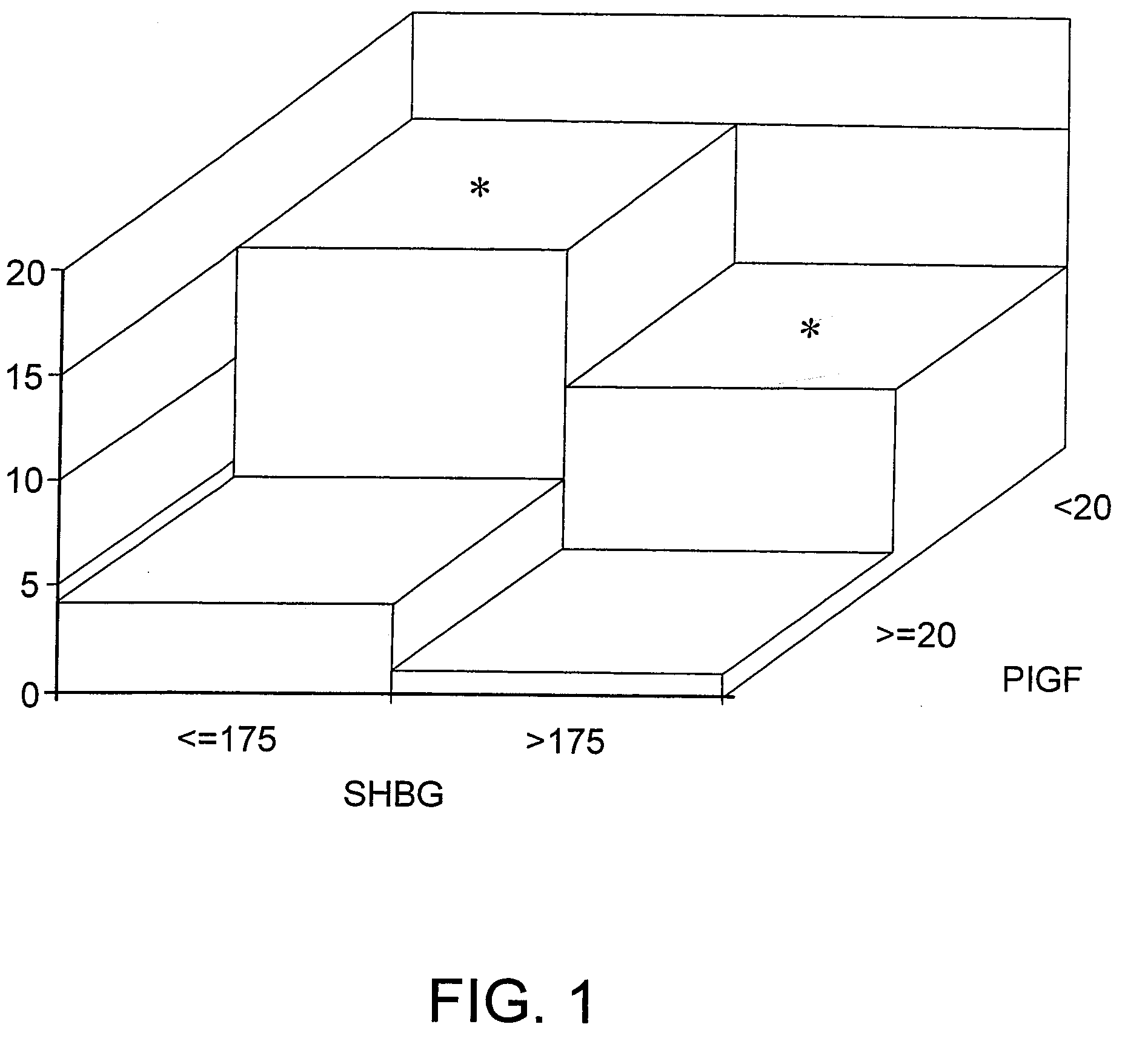
SHBG and P1GF Assays
[0137]Study Population and Data Acquisition
[0138]A prospective nested case-control study of patients who had enrolled in the Massachusetts General Hospital Obstetrical Maternal Study (MOMS) was performed. In brief, the MOMS cohort was established in 1998 for the prospective study of early gestational risk factors for adverse outcomes that occur later in pregnancy. For this study, consecutive women with singleton gestations between Jun. 1, 2001 and May 1, 2003 who enrolled in the MOMS cohort at approximately 12 weeks of gestation, and who delivered after 20 weeks were eligible for inclusion. All subjects provided written informed consent.
[0139]The electronic medical record, which is the medical record used by the clinical staff, provides clinical and demographic data that prospectively details the events of pregnancy through the early postpartum period. Specific information obtained from the electronic medical record included age, race, height, weight, blood press...
example 2
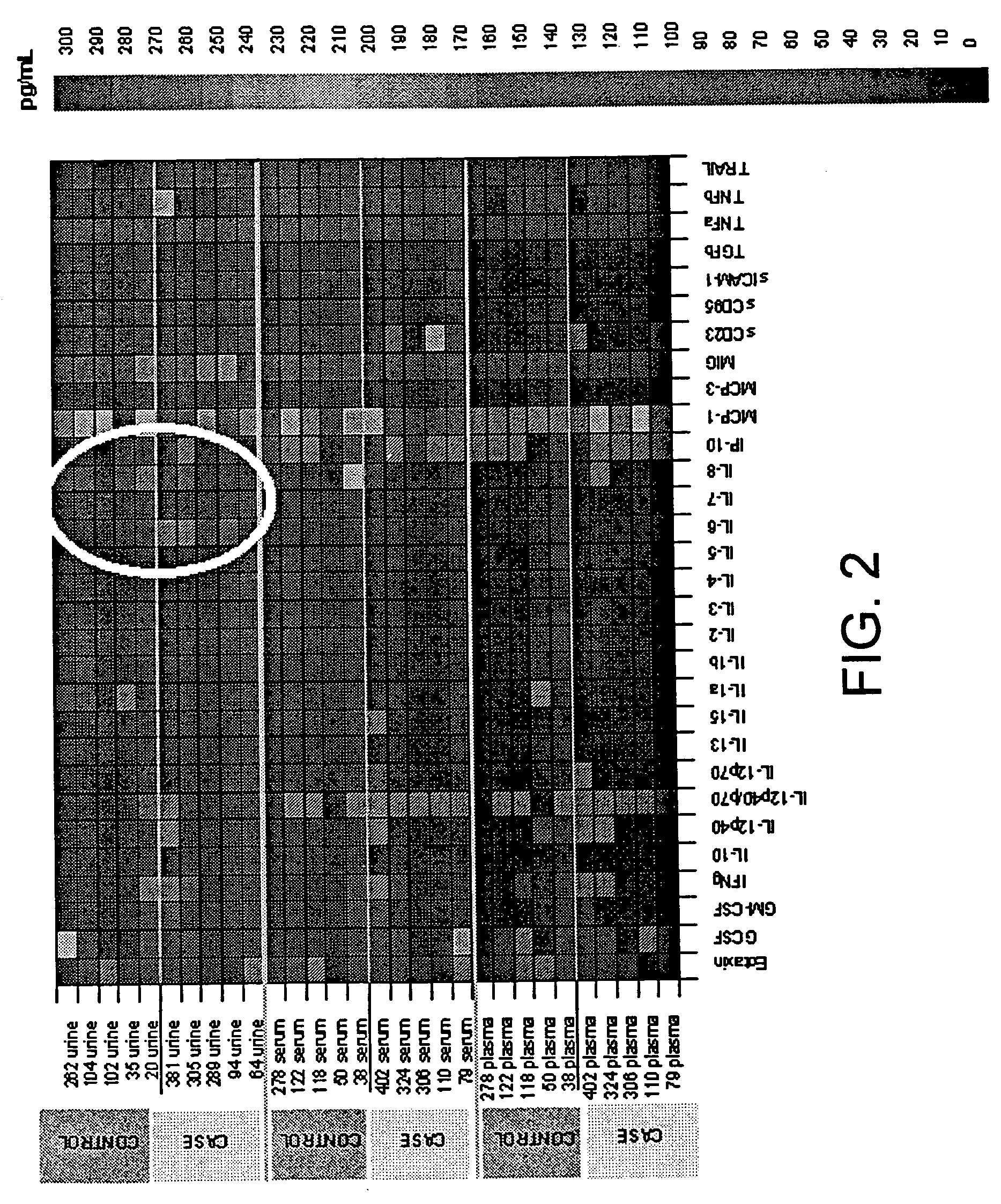
Cytokine Assays
[0150]The present invention demonstrates that the alteration of a single cytokine or growth factor can be used to identify subjects having, or predisposed to having, preeclampsia, GDM or GH. Urine, plasma, and serum samples were tested for cytokine levels using a cytokine array (Zyomyx®). The array permits the quantitative analysis of 30 cytokines and chemokines, including IL-1α, IL-3, IL-6, IL-10, IL-12 (p70), TNF-A, MCP-1, CD95 (sFas), IP-10, GM-CSF, IL-1β, IL-4, IL-7, IL-12 (p40), IL-13, TNF-β, MCP-3, MIG, CD23, GCSF, IL-2, IL-5, IL-8, IL-12 (p40 / p70), IL-15, Eotaxin, TRAIL, sICAM-1, TGF-β and IFN-γ, using a sample volume of approximately 40 μl of complex biological fluids, such as serum or urine. The data quality is comparable to standards established by ELISA assays. A spike / recovery analysis in urine was carried out and recovery of cytokines in urine (r=0.92) was determined. All subjects had normal renal function, thus it was unlikely that urea interfered with t...
example 3
Growth Factor Assays
[0155]In conjunction with cytokine levels, the present studies provide information regarding the levels of growth factors in urine and blood samples. Serum and urine samples from 16 weeks of gestation in 15 subjects (5 who developed GDM, 5 PE, and 5 controls) were tested with commercially available ELISA kits for exemplary growth factors sFlt-1, free-VEGF, and free-P1GF (R&D Systems). These ELISA kits have inter-assay and intra-assay CV's of <10%. All assays were performed in duplicate, and the averages are reported in FIGS. 5 and 6. Free-VEGF levels were undetectable, consistent with low VEGF levels generally detected at term.
[0156]The data derived from serum samples shown in FIG. 5 indicates that, compared to control women, women who develop GDM have low free P1GF and slightly elevated sFlt-1 levels at 16 weeks of gestation. Moreover, women who subsequently develop PE have even lower free P1GF and higher sFlt-1 levels at this same time period. The balance of an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com