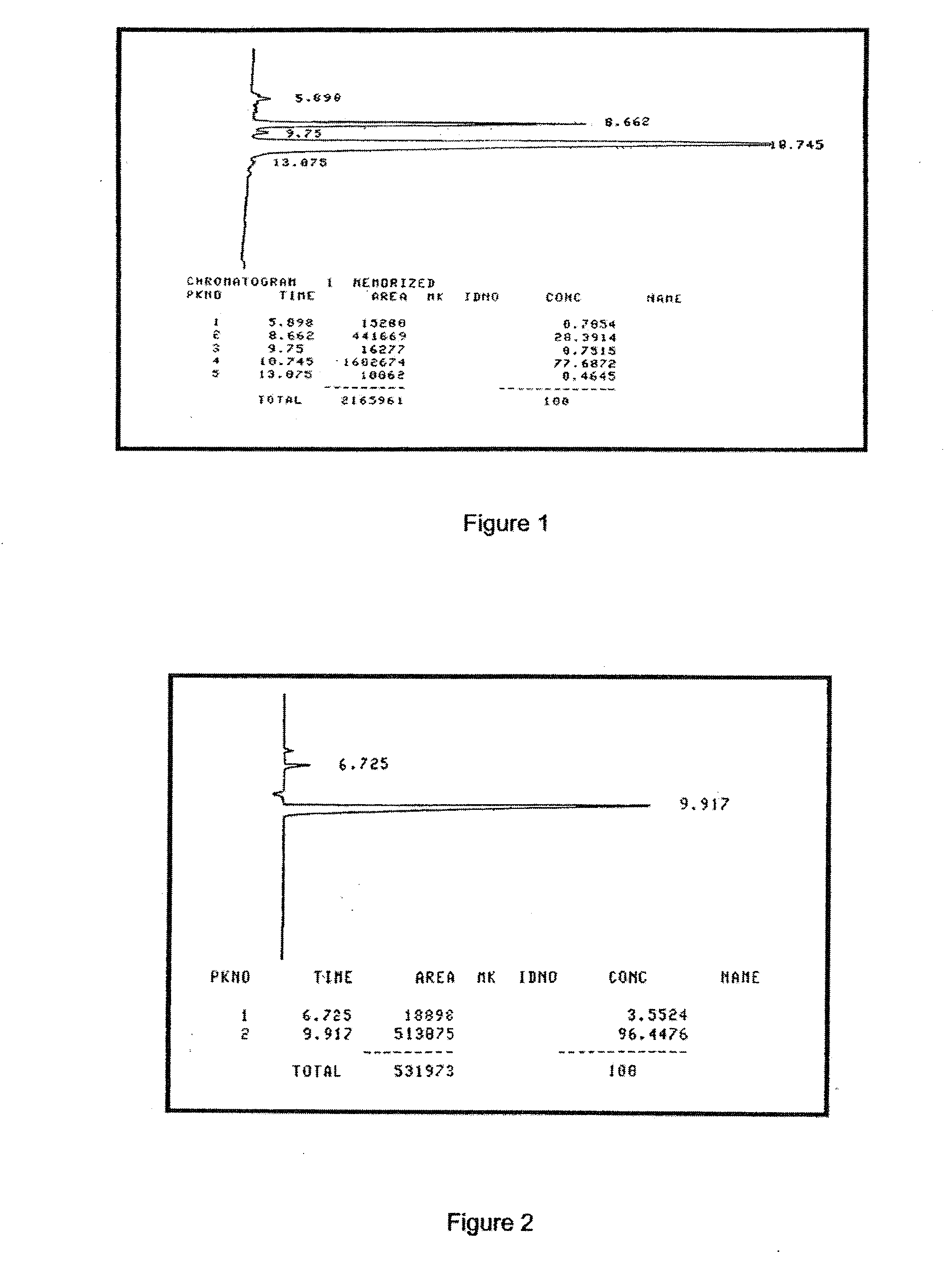
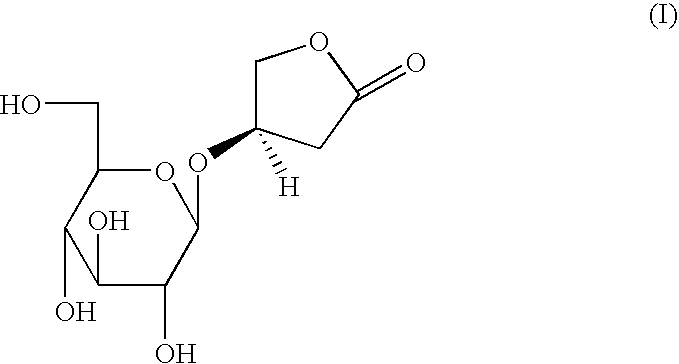
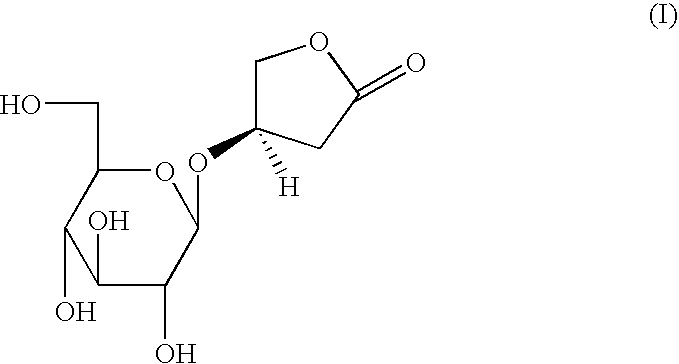
Aqueous extracts of anoectochilus spp. kinsenoside-comprising pharmaceutical compositions useful for hepatoprotection and uses of the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
CCl4-induced Chronic Hepatitis in Mice
[0047]Male ICR mice were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and were used for experiments when they reached 24 to 26 g body weight. Chronic hepatitis was induced in mice by oral administration of 0.1 mL of CCl4 (diluted 1:19 in olive oil) per 10 g body weight twice a week for 3, 8 or 9 weeks. On the days of the CCl4 treatment, the time interval between CCl4 and drug administrations was 5 hours to avoid absorption interference. After the blood was drawn at the end of the experimental period, all mice were sacrificed at the same time and the liver and spleen were quickly removed and weighed after washing with cold normal saline and blotting dry. The largest lobe of liver was weighed and then completely dried at 100° C. for determination of the hydroxyproline content according to the method designed by Neuman and Logan (Neuman R E and Logan M A, J Biol Chem, 1950, 184: 299-306). Dried liver tissue (approx. 60 mg) ...
experiment i
[0048]Chronic hepatitis was induced in mice by CCl4 administration for 3 weeks. Since AFEE did not dissolve in water, it was suspended in polyvinylpyrrolidone (PVP). Therefore, the experiments were divided into two parts. For part I, mice were divided into three groups, those that received CCl4 with H2O, CCl4 with AFE (2000 mg / kg) or CCl4 with AFEW (1500 mg / kg) p.o. daily for 3 weeks. The control group received olive oil (vehicle for CCl4) with H2O. In part II, the mice were divided into two groups, those that received CCl4 with PVP or CCl4 with AFEE (500 mg / kg) p.o. daily for 3 weeks. The control group received the olive oil vehicle with PVP. After 1 week of CCl4 treatment, blood was collected via the retro-orbital sinus of mice for serum GPT assays.
[0049]The results are shown in Table 2. CCl4 treatment caused hepatocellular damage in mice, as indicated by an increase in plasma GPT activity after 1 and 3 weeks of CCl4 administration. Mice that were treated daily with AFE (2000 mg / k...
experiment ii
[0050]Chronic hepatitis was induced in ICR mice by CCl4 administration for 8 weeks. Since AFEW-4 did not dissolve in water, it was suspended in PVP. The experiment was divided into two parts. In part I, mice were divided into six groups, those that received CCl4 with H2O, CCl4 with AFEW (1500 mg / kg), CCl4 with AFEW-1 (1055 mg / kg), CCl4 with AFEW-2 (130 mg / kg), CCl4 with AFEW-3 (50 mg / kg) or CCl4 with AFEW 5 (190 mg / kg) p.o. daily for 8 weeks. The control group received the olive oil vehicle with H2O. In part II, mice were divided into two groups those that received CCl4 with PVP or those that received CCl4 with AFEW-4 (75 mg / kg) p.o. daily for 8 weeks. The control group received the olive oil vehicle with PVP.
[0051]An animal model of chronic hepatitis induced in mice by oral administration of CCl4 twice a week for 8 weeks was used to investigate the hepatoprotective effect of AFEW-1 to AFEW-5. The result is shown in Table 4. Compared to the control group in week 8, the CCl4-treated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com