ASSAY FOR MEASURING FACTOR VIIa-ANTITHROMBIN COMPLEXES
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
ELISA Assay for Measuring the Level of Factor VIIa-AT Complexes in Plasma Using p-NPP Substrate (With Use of Blocking Agent)
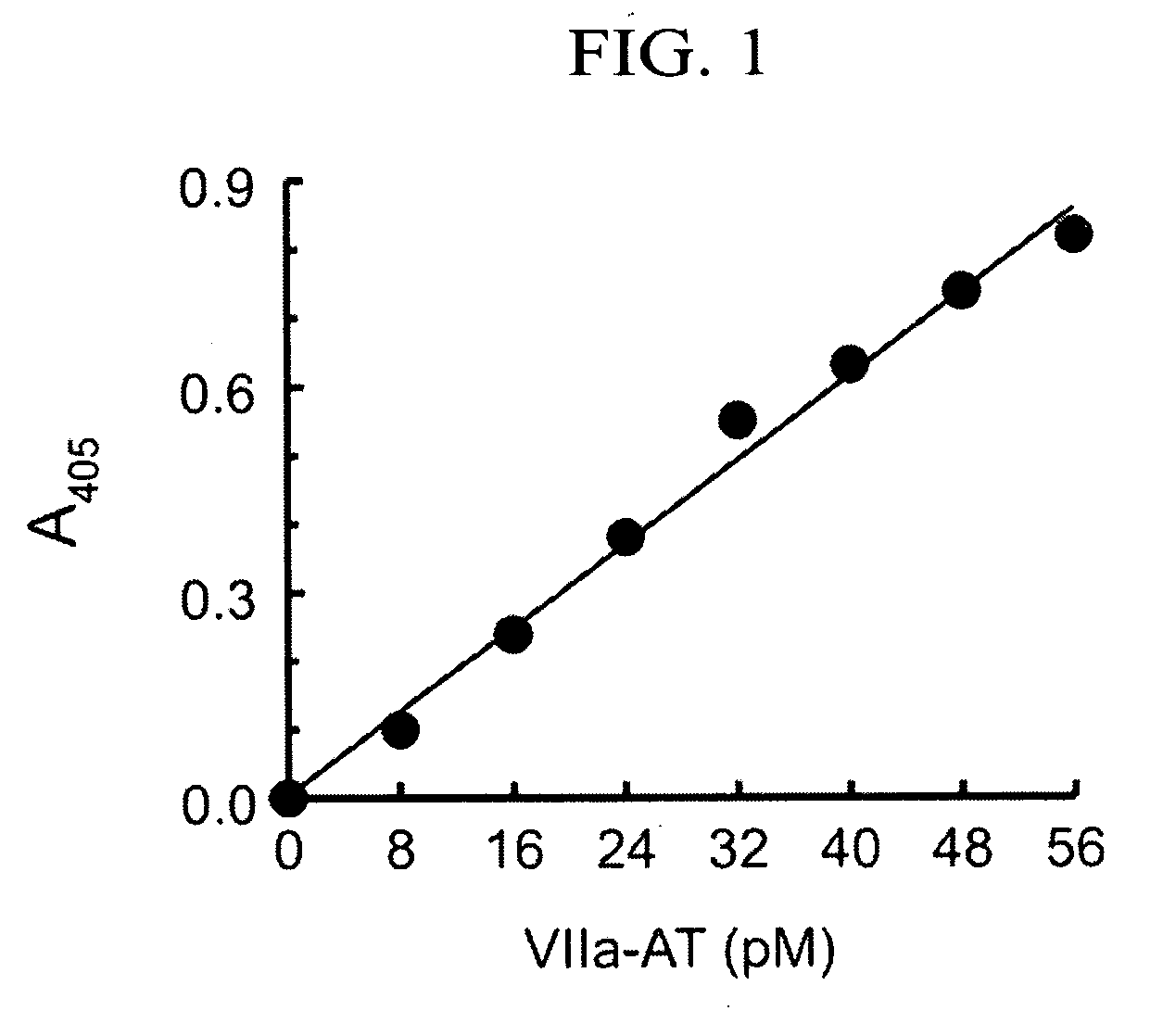
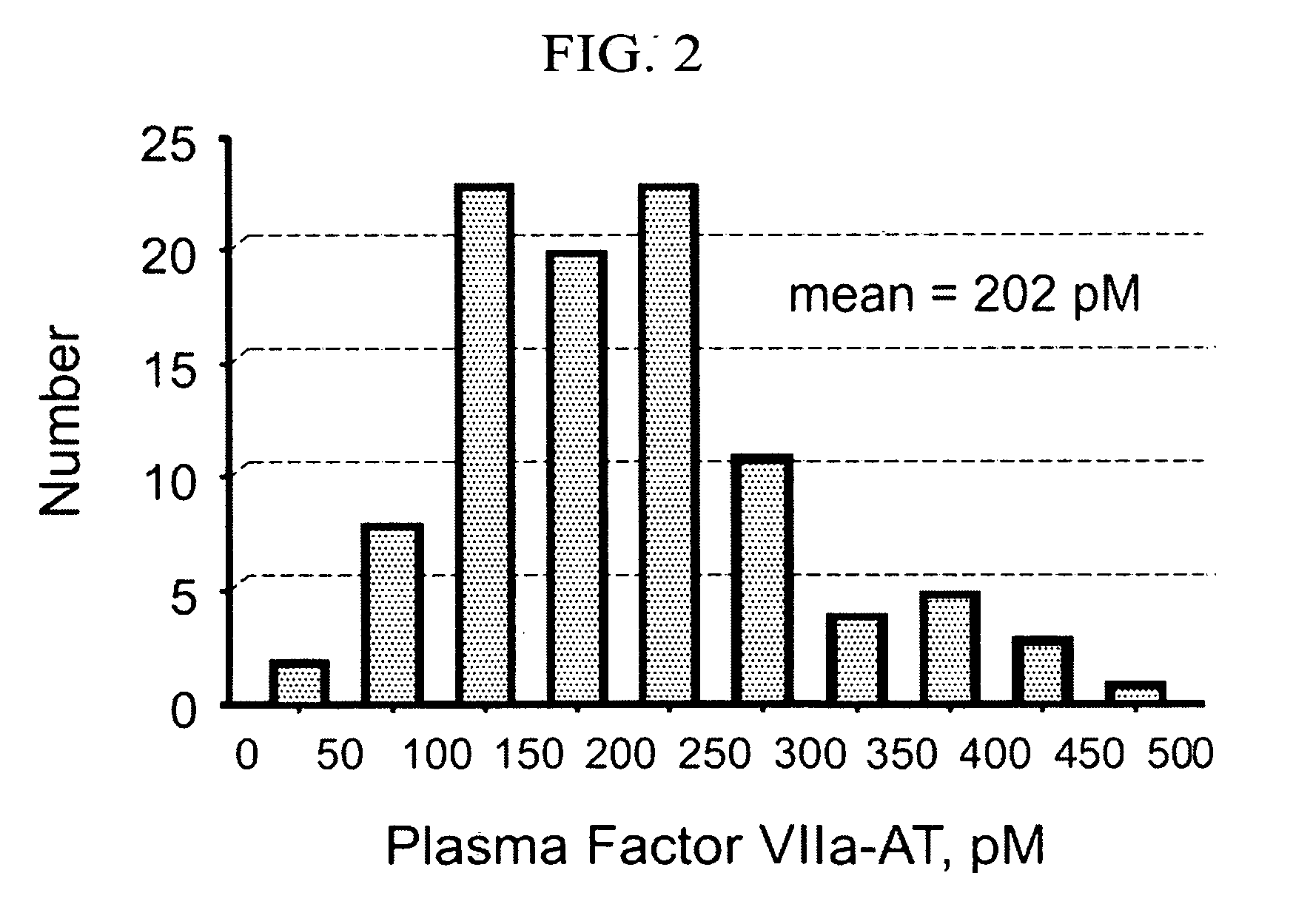
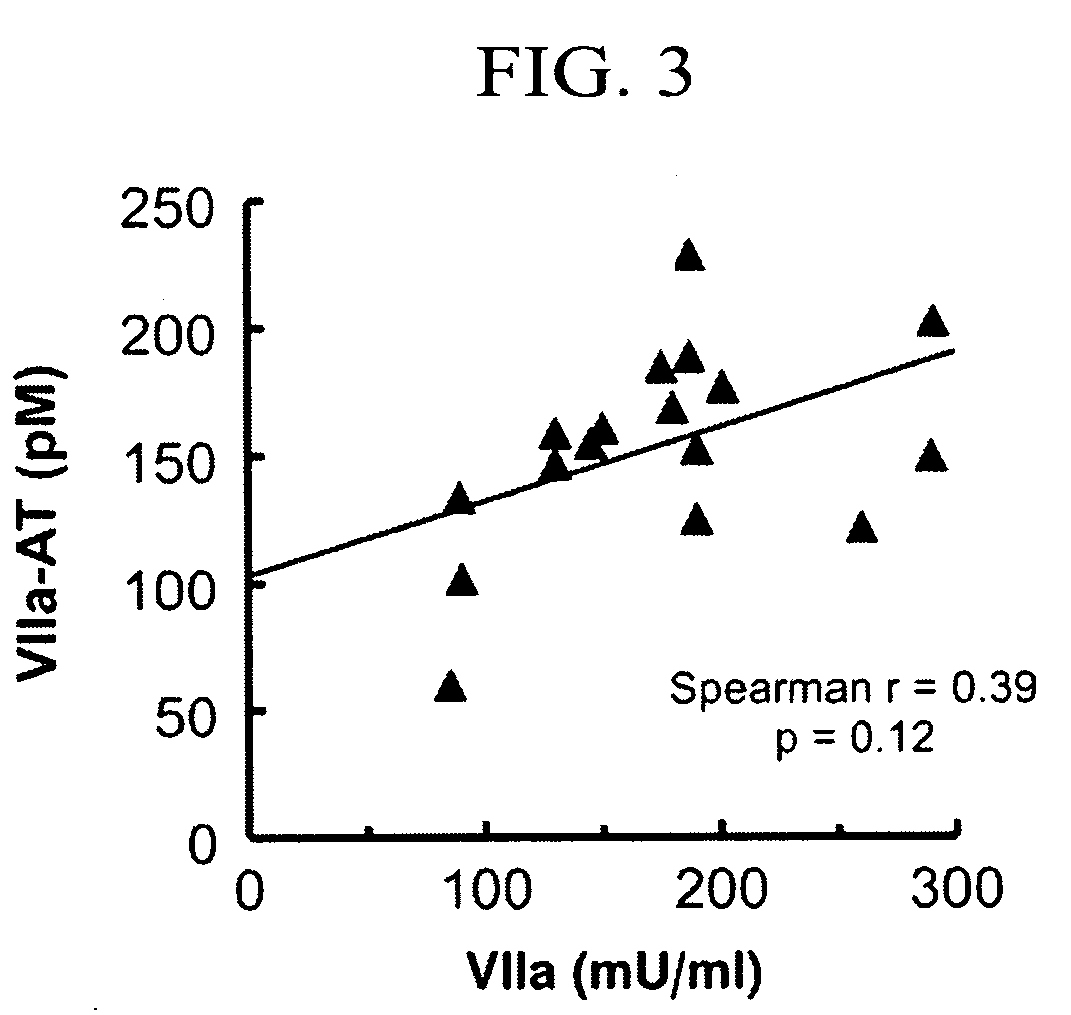
[0039]The ELISA assay was used to measure the concentration of factor VIIa-antithrombin (factor VIIa-AT) complexes in human plasma and other samples.
Preparation of Factor VIIa-AT Complexes for Standardizing the Factor VIIa-AT ELISA
[0040]A 1.1 ml solution of 100 nM factor VIIa [Factor VIIa (recombinant); catalog #407recB; American Diagnostica, Inc., Greenwich, Conn.] and 150 nM sTF [recombinant soluble tissue factor protein produced by methods known in the art] was prepared in HBSA / calcium [HBSA / calcium: 30 mM Hepes buffer, pH 7.4, 100 Mm NaCl, 5 mM CaCl2, 0.1% w / v bovine serum albumin, and 0.1% w / v sodium azide and stored at 4° C.].
[0041]A 0.1 ml aliquot was removed and held on ice to form factor VIIa-sTF complexes.
[0042]To the remaining 1.0 ml reaction mixture, heparin (catalog #H3393; Sigma Chemical Co. St. Louis, Mo.) was added to a final concentration of 1 ...
example 2
ELISA for Factor VIIa-Antithrombin (VIIa-AT) Complexes (Without Blocking Agent)
[0053]Materials. The materials used in this example were as follows:[0054]Anti-Factor VII Monoclonal Antibody—a calcium independent murine monoclonal antibody raised against human factor VII in the Morrissey lab, the hybridoma cells deposited as ATCC No. PTA-3497.[0055]Anti-Human Antithrombin III Rabbit polyclonal antibody)—DAKO catalog number A0296.[0056]Bovine Serum Albumin—Calbiochem catalog number 12659 (“Albumin, bovine serum, fraction V, low heavy metals”)[0057]Donkey Anti-rabbit IgG (H+ L), (min.times.BvChGtGuHaHsHnMsRtSh Sr Prot), Immunopure.RTM., Alkaline Phosphatase Conjugated—Pierce catalog number 31345.[0058]ELISA plates—Flat bottom Costar EIA / RIA Plates from Corning Inc. (Corning / Costar number 9018).[0059]Factor VII-deficient plasma—Congenital factor VII-deficient plasma (citrated) from George King BioMedical, Inc. (www.kingbiomed.com)[0060]pNPP (p-nitrophenyl phosphate)—Sigma catalog number ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com