Skin Retention Device for a Medical Jet Injection Unit
a technology of skin retention and injection unit, which is applied in the direction of jet injection syringes, injection syringes, medical syringes, etc., can solve the problems of unfavorable high injection force, risk of damage, and unpredictable plasma insulin levels, so as to reduce the likelihood of the nozzle moving relative to the skin during injection and achieve a greater degree of stretching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036]When in the following terms as “distal”, “proximal” and “radial” or similar relative expressions are used, these only refer to the appended figures and not necessarily to an actual situation of use. The shown figures are schematic representations for which reason the configuration of the different structures as well as there relative dimensions are intended to serve illustrative purposes only.
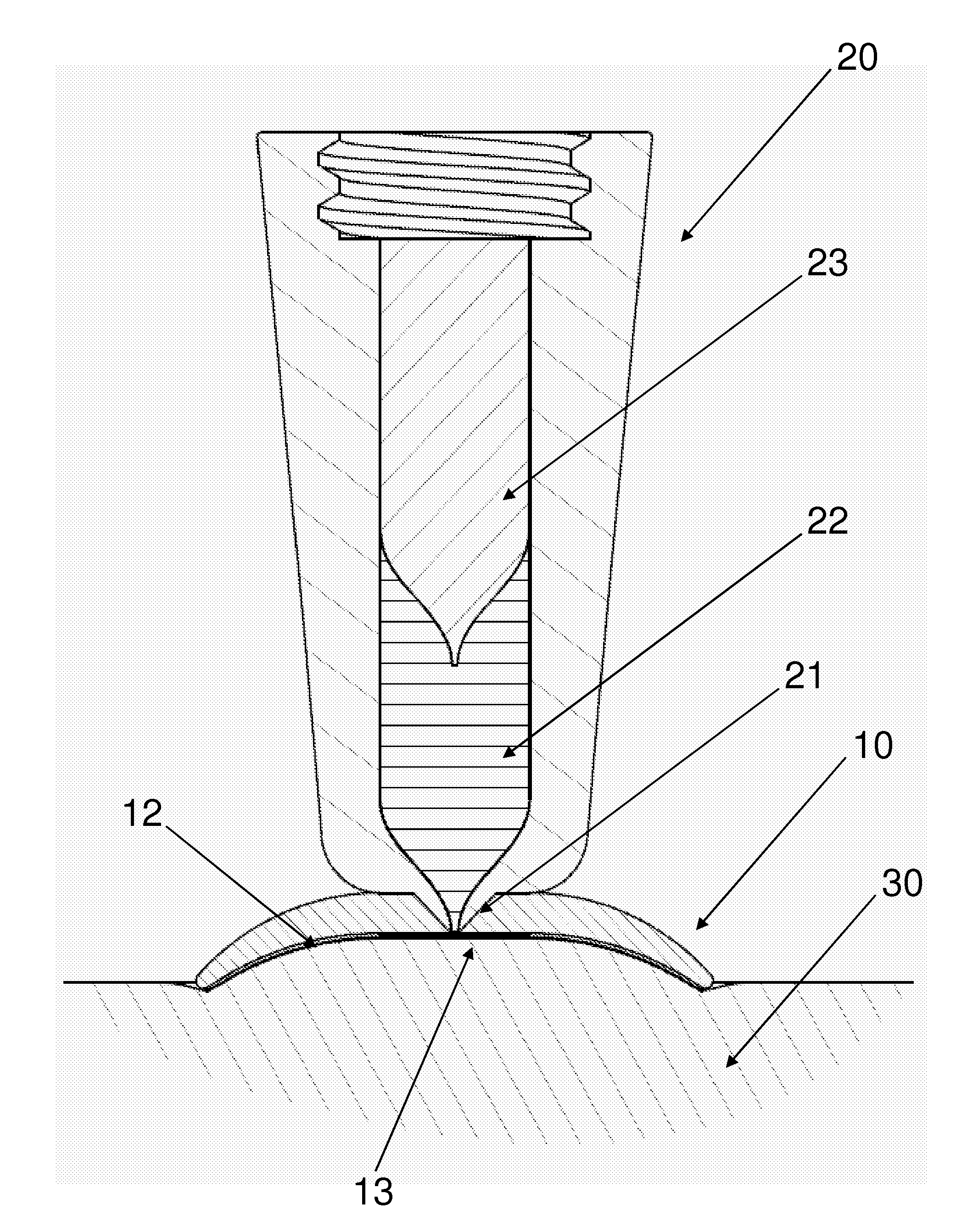
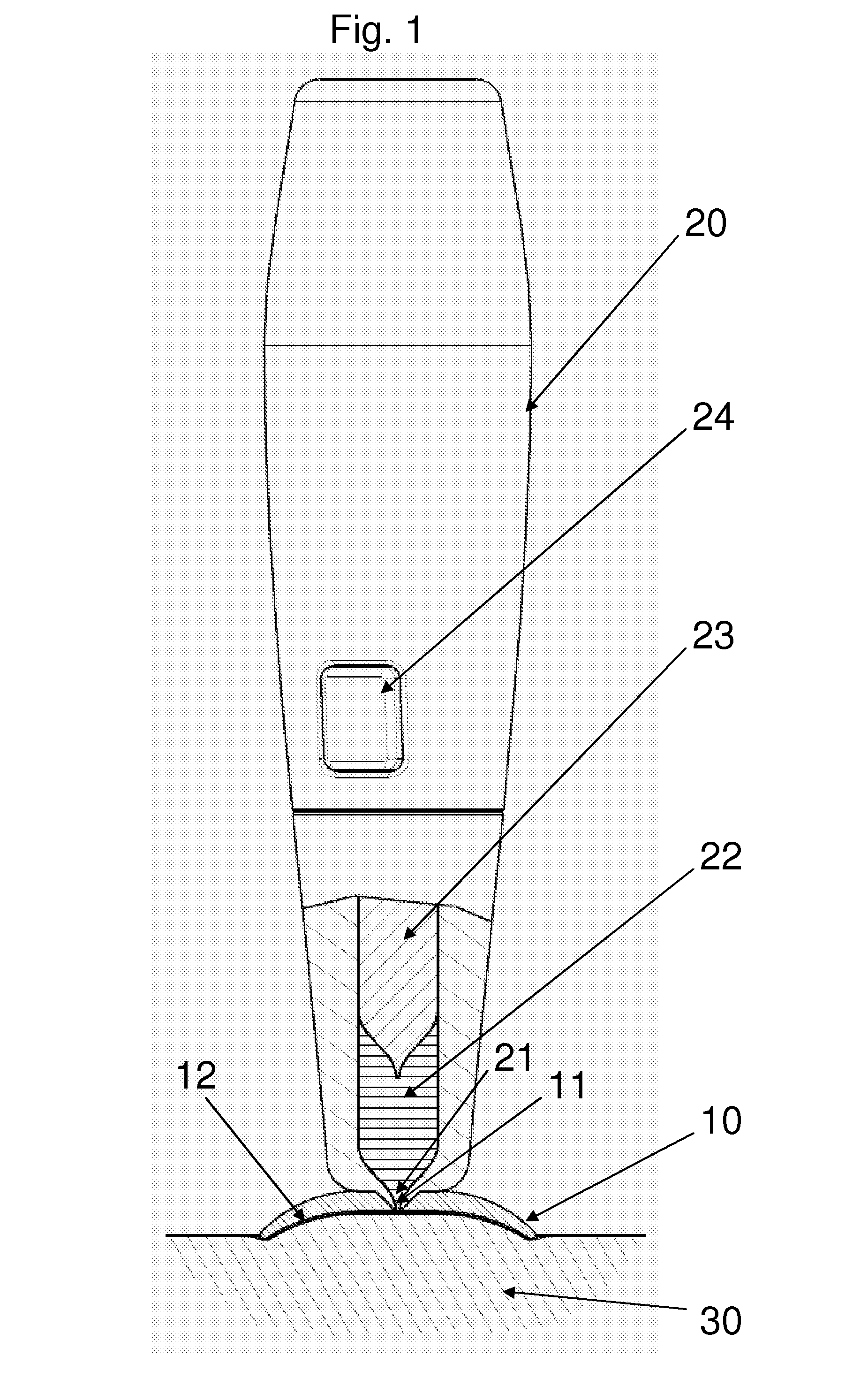
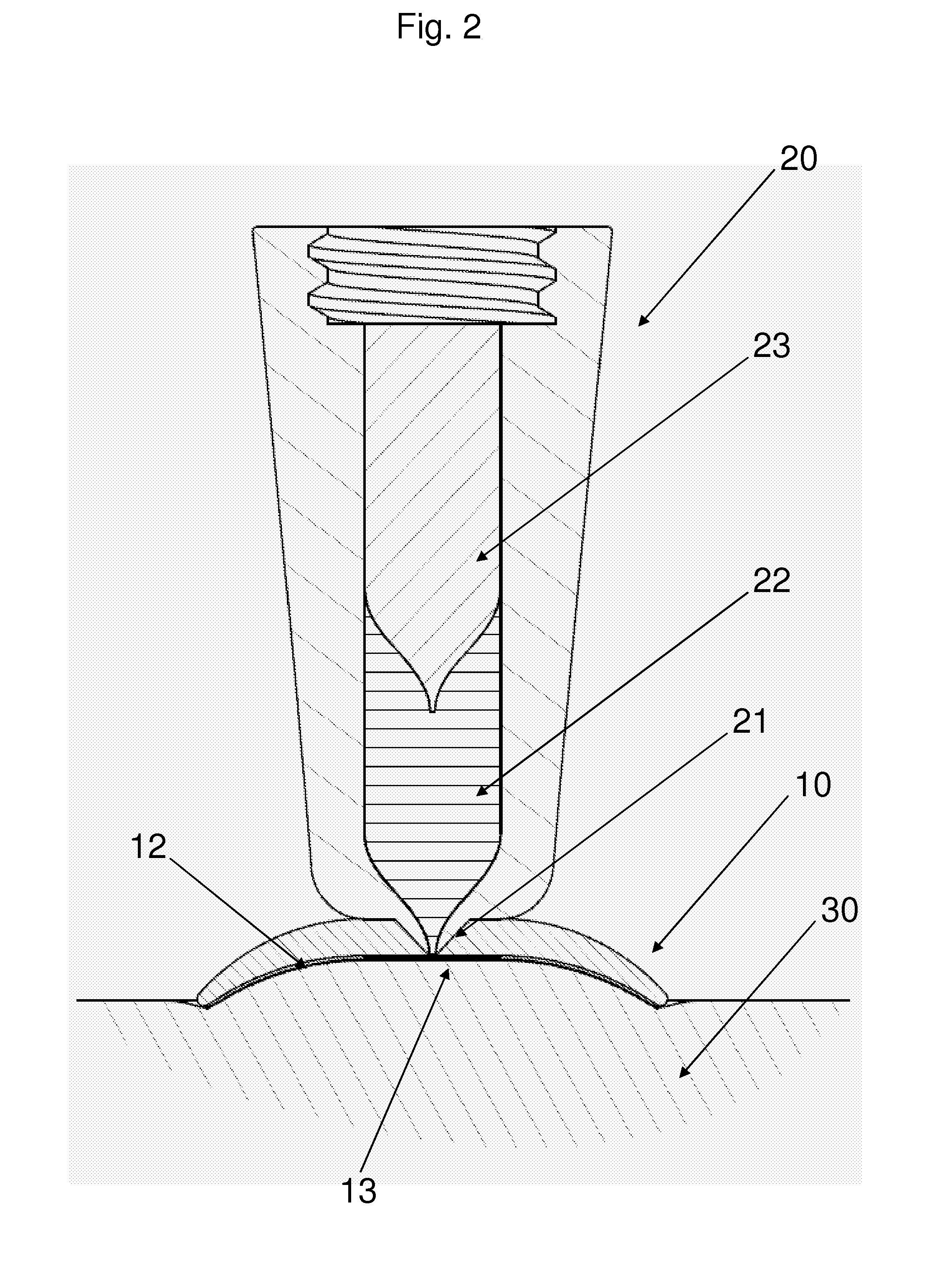
[0037]FIG. 1 shows a side view of a jet injection unit 20 and a skin retention device 10 connected thereto. The lower part of the unit is shown in sectional view to show the piston 23 slidingly arranged in a housing thereby defining a variable-volume impulse chamber 22 in flow communication with the aperture through a nozzle conduit 21. In the shown embodiment the impulse chamber is adapted for being filled with a liquid drug by suction through the nozzle conduit by moving the piston 23 proximally, however the impulse chamber unit 22 may also be provided with an opening in either the hous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com