Therapeutic peptidomimetic macrocycles
a technology of peptidomimetic macrocycles and macrocycles, which is applied in the direction of peptide/protein ingredients, metabolism disorders, immunological disorders, etc., can solve the problems of limited side effects, side effects, cost, and difficulty in treating cell proliferative disorders, so as to reduce the risk of cancer survival, less sensitive to treatment, and less sensitive to treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Peptidomimetic Macrocycles of the Invention
[0343]α-helical BID and BIM peptidomimetic macrocycles were synthesized, purified and analyzed as previously described (Walensky et al (2004) Science 305:1466-70; Walensky et al (2006) Mol Cell 24:199-210) and as indicated below. The macrocycles used in this study are shown below. The corresponding uncrosslinked polypeptides are indicated as “WT Sequence” and represent the natural counterparts of the peptidomimetic macrocycles of the invention.
Calculat-Calcu-FoundMacro-WTed m / zlated m / zm / zcycleSequenceSequence(M + H)(M + 3H)(M + 3H)SP-1BID-BH3Ac-DIIRNIARHLA$VGD$NleDRSI-NH22438.40813.47813.7SP-2BID-BH3Ac-DIIRNIARHLA$VED$NleDRSI-NH22510.42837.48837.25SP-3BID-BH3Ac-DIIRNIARHLAQVGDSNleDRSI-NH22403.32801.78801.89SP-4BIM-BH3Ac-IWIAQELR$IGD$FNAYYARR-NH22646.43882.82883.15SP-5BIM-BH3Ac-IWIAQELR$IED$FNAYYARR-NH22718.45906.82906.9SP-6BIM-BH3Ac-IWIAQELRRIGDEFNAYYARR-NH22681.41894.47894.69SP-7BID-BH3Pr-RNIARHLA$VAibD$NleDRSI-NH22139.25713....
example 2
Cell Viability Assays
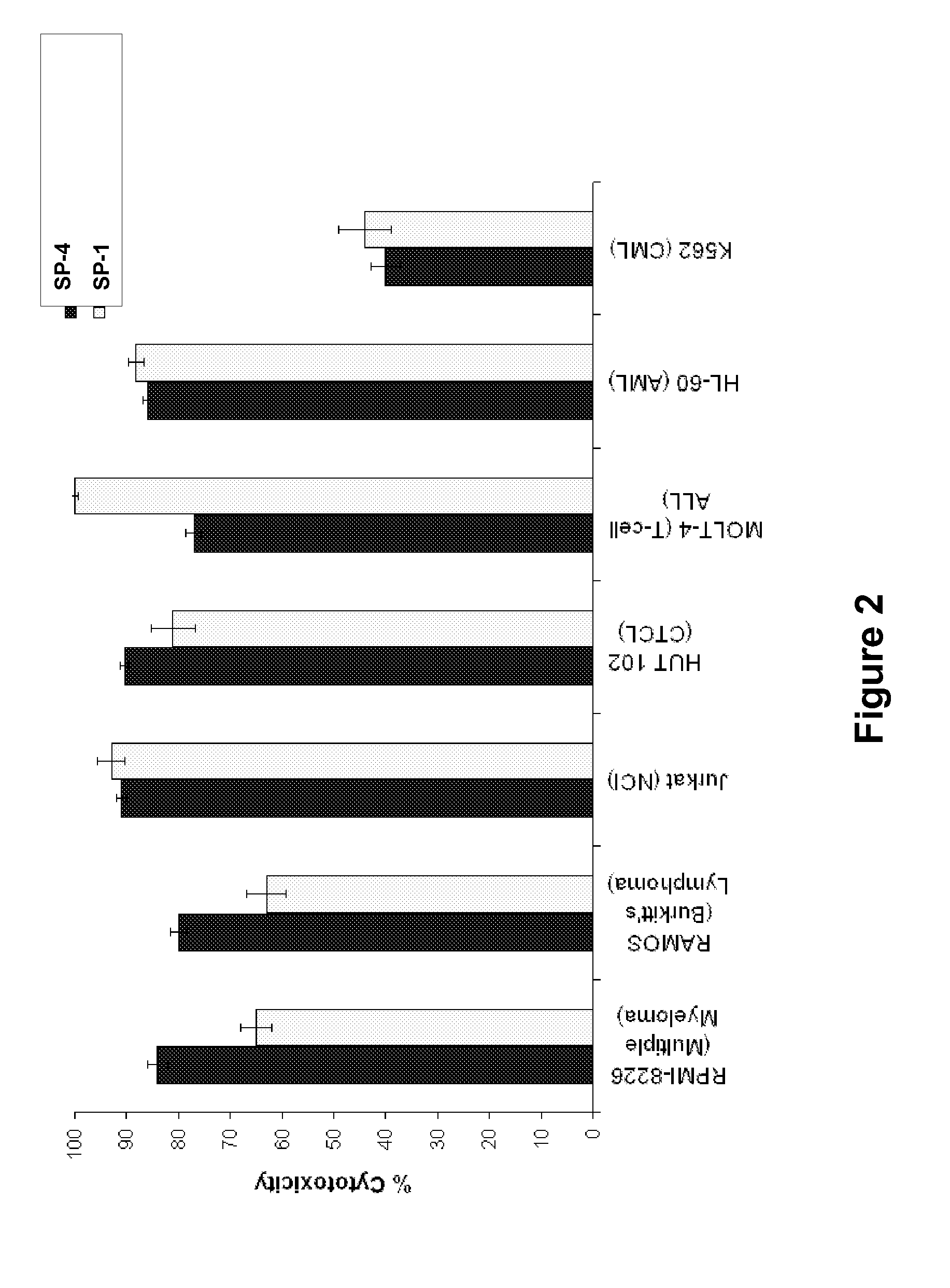
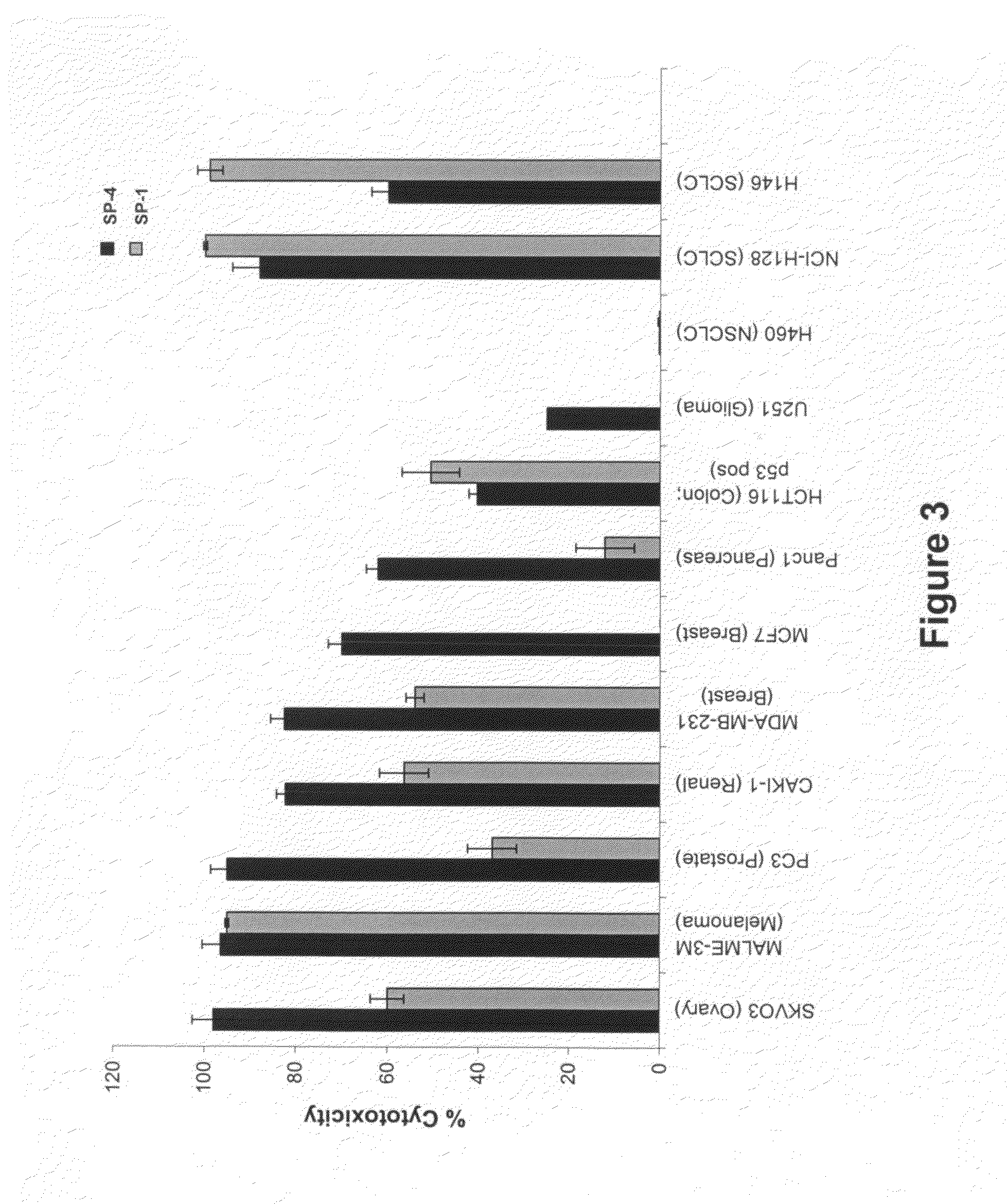
[0348]Cell viability assays shown in FIGS. 1-32 were performed according to the following protocol. Tumor cell lines were grown in specific serum-supplemented media (growth media) as necessary. A day prior to the initiation of the study, cells were plated at optimal cell density (15,000 to 25,000 cells / well) in 200 μl growth media in microtiter plates. The next day, cells were washed twice in serum-free / phenol red-free RPMI complete media (assay buffer) and a final volume of 100 μl assay buffer was added to each well. Human peripheral blood lymphocytes (hPBLs) were isolated from Buffy coats (San Diego Blood Bank) using Ficoll-Paque gradient separation and plated on the day of the experiment at 25,000 cells / well.
[0349]Peptidomimetic macrocycles were diluted from 1 mM stocks (100% DMSO) in sterile water to prepare 400 μM working solutions. The peptidomimetic macrocycles and controls were then diluted 10 or 40 fold or alternatively serially two-fold diluted in assa...
example 3
BrdU Cell Proliferation Assay
[0356]hPBLs isolated from two different donors were stimulated or not with 5 μg / ml PHA, 1 μM Ionomycin and 1 μg / ml LPS and treated with either 5 or 20 μM of SP-1 in assay buffer. 1 μM Rapamycin was used as a positive control to inhibit BrdU incorporation. The cells were incubated for 48 hrs under the conditions indicated in FIG. 17. BrdU incorporation was assayed by ELISA according to manufacturer's instructions (Roche, catalog number 11444611001). In FIG. 7, the Y axis shows OD=Absorbance (A405 nm / A492 nm.)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com