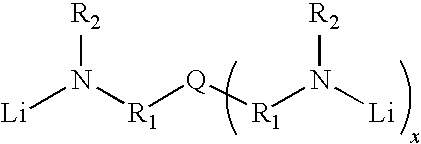
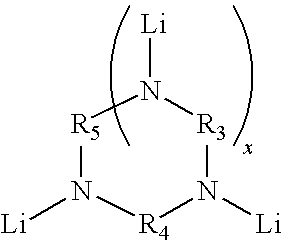
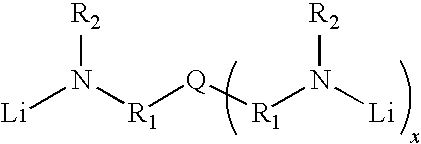
Novel multifunctional initiators for anionic polymerization and polymers therefrom
a multifunctional, anionic polymerization technology, applied in the field of compounds, can solve the problems of low initiator concentration, bi-modal initiation, pre-reaction steps to synthesize,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0089]To a dried 28-oz glass bottle was added 83.3 g of hexane, and 166.7 g of 18.0 wt % butadiene in hexane, followed 0.34 ml of 4,4′-trimethylenedipiperidine solution (1.0 M in toluene) and 0.47 ml of n-BuLi (1.60M) in hexane by hypodermic syringe. The bottle was agitated and heated at 50° C. for 1.5 hr. After polymerization, the living cement was quenched by injection with 1.5 ml of isopropanol (1-PrOH), treated with an antioxidant (3 ml of 2 wt % di-t-butyl-p-cresol in hexane), coagulated in i-PrOH, then vacuum dried. Characterizations were performed, and the result was listed in Table 1, wherein Bd means butadiene; 1,2-Bd %, cis % of 1,4-Bd and trans of 1,4-Bd are analyzed by FT-IR; Mn means number average molecular weight; Mw means weight average molecular weight; Mp means peak molecular weight; PDI means polydispersity index (Mw / Mn); Tg means glass transition temperature. The molecule weight of the polymer in the example was determined by using a Waters Model 150-C GPC.
TABLE ...
example 2
[0090]The experimental in example 1 was repeated, however, with varied amount of randomizer of 2-bis(2′-tetrahydrofuranyl)propane (OOPS) and the effect of randomizer of 2-bis(2′-tetrahydrofuranyl)propane on polymer Tg (° C.) was listed in Table 2.
TABLE 2OOPS:“Li” (molar ratio)Tg (° C.)0.00−94.230.15−46.520.25−39.240.35−33.89
example 3
[0091]To a dried 28-oz glass bottle was added 91.7 g of hexane, and 208.3 g of 21.6 wt % butadiene in hexane, followed 0.61 ml of tris[2-(methylamino)ethyl]amine solution (1.0 M in toluene), 1.4 ml of N,N,N′,N′-tetramethylethylenediamine (1.0M in hexane), and 1.15 ml of n-BuLi (1.60M) in hexane by hypodermic syringe. The bottle was agitated and heated at 50° C. for 1.5 hr. After polymerization, the living cement was quenched by injection with 1.5 ml of isopropanol (i-PrOH), treated with an antioxidant (3 ml of 2 wt % di-t-butyl-p-cresol in hexane), coagulated in i-PrOH, then vacuum dried. Characterizations were performed, and the result was listed in Table 3.
TABLE 3MnMwMpCouplingID(g / mol)(g / mol)(g / mol)PDI(%)Tg (° C.)Example 36985872166738561.0330−28.84
PUM
| Property | Measurement | Unit |
|---|---|---|
| tan δ | aaaaa | aaaaa |
| length scale | aaaaa | aaaaa |
| volume percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com