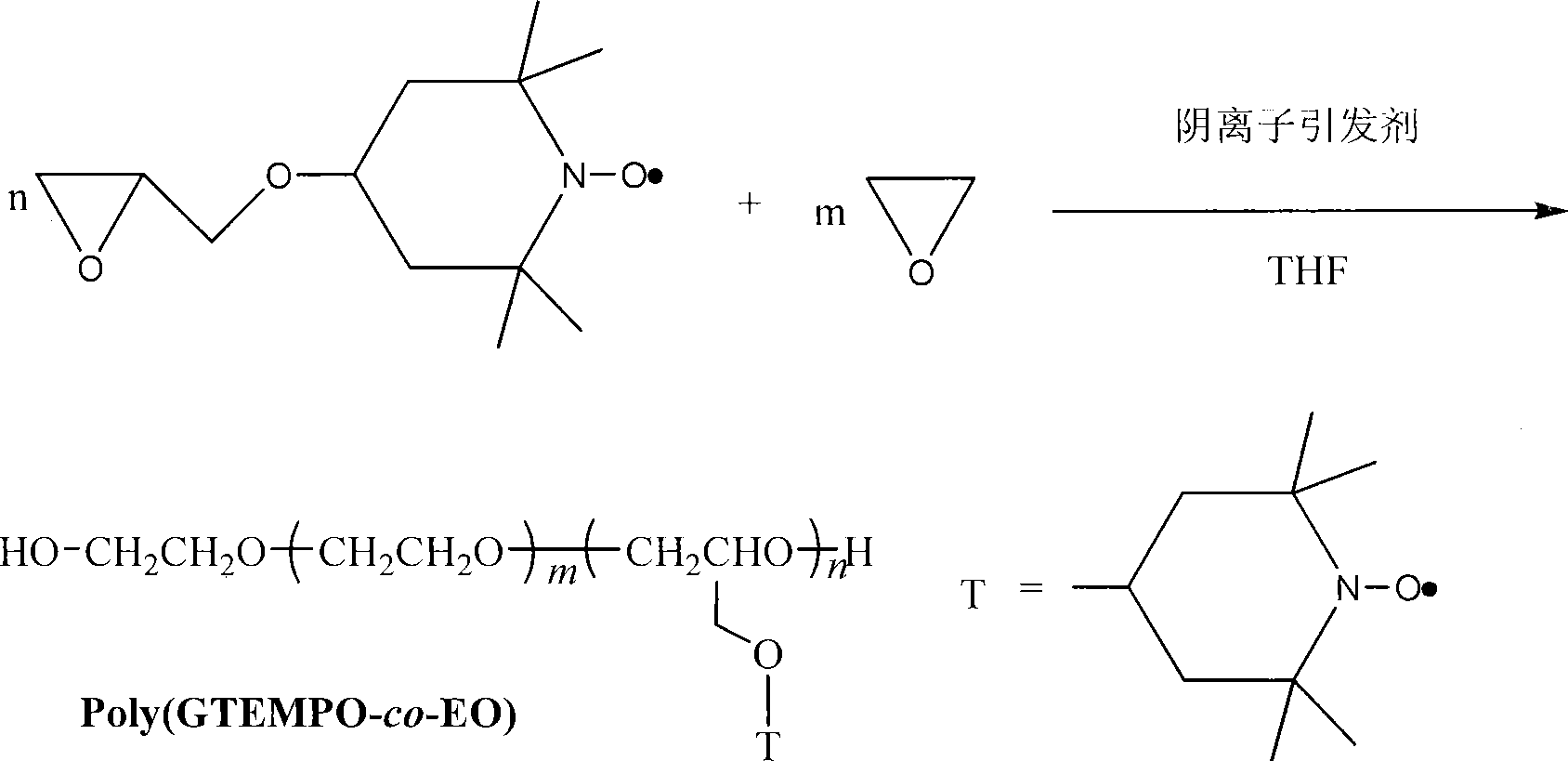Amphiphilic macrocyclic polymer and preparation method
An amphiphilic and polymer technology, which is applied in the field of amphiphilic macrocyclic polymers and their preparation, can solve the problems of single chemical structure of side chains, limited number of side chains, no polymers found, etc., and achieve clear synthesis routes, The method is simple and the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] c-PEO-g-PS
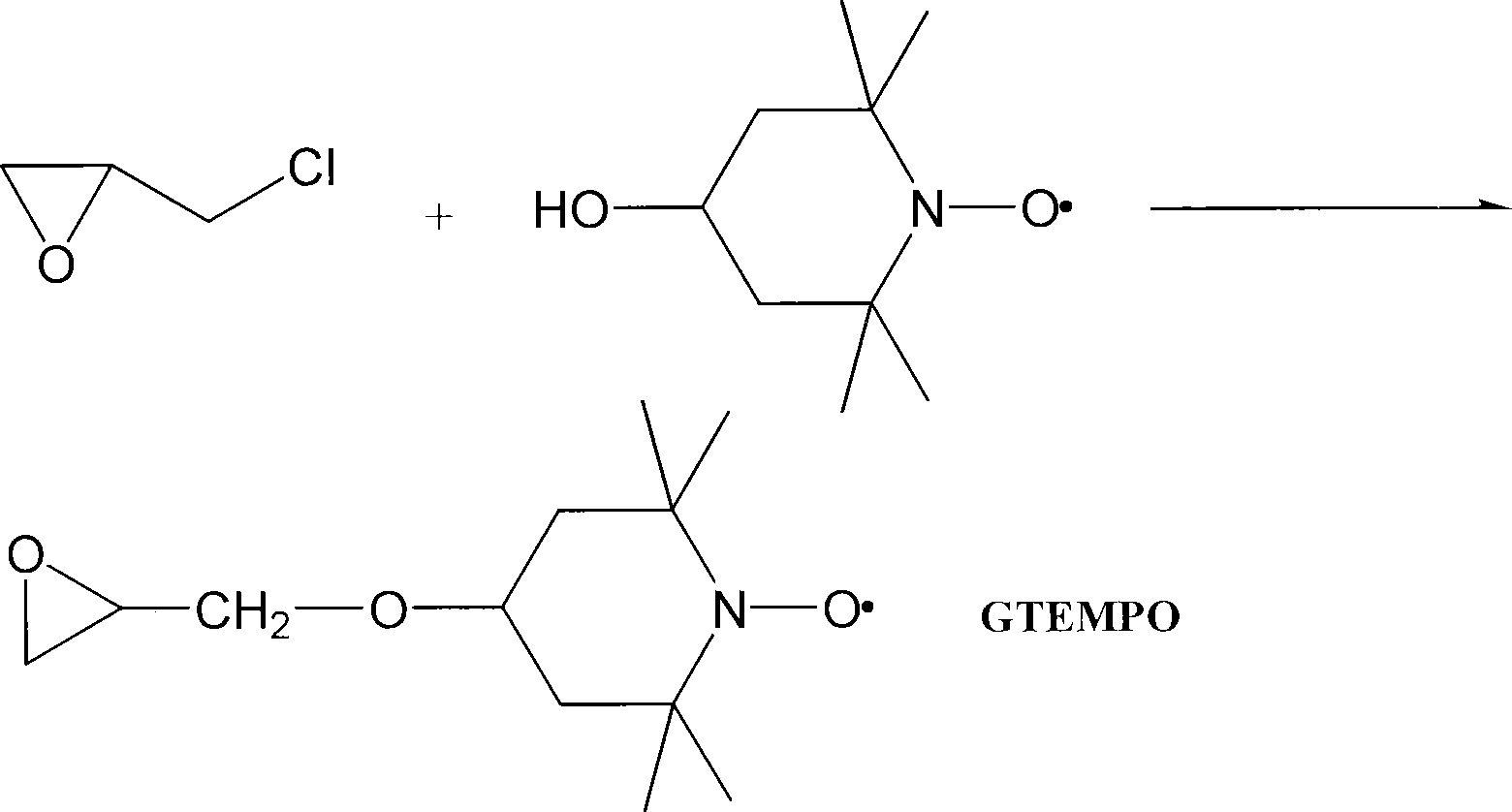
[0042] 1. Preparation of 4-glycidyl-2,2,6,6-tetramethylpiperidine nitroxide radical (GTEMPO)
[0043] Add 340mL 50% (v / v) sodium hydroxide solution to a 1000mL three-necked flask, add 7g tetra-n-butylammonium hydrogensulfate (TBAHS) into the flask, keep an ice-water bath, so that the reaction temperature does not exceed 25°C. While stirring mechanically, 210mL of epichlorohydrin was added through the dropping funnel within 30min. After keeping the reaction in the ice-water bath for 2 hours, 86 g of 4-hydroxy-2,2,6,6-tetramethylpiperidine nitroxide was added in batches within 1 hour. After the reaction was carried out for 24 hours, the reaction mixture was poured into an ice-water mixture (1.5 L), and the aqueous phase was repeatedly extracted with ether until the aqueous layer turned pale yellow. The ether solutions extracted several times were combined and washed with anhydrous MgSO 4 After drying for 3 h, CaH 2 Let dry overnight. After diethyl ether ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com