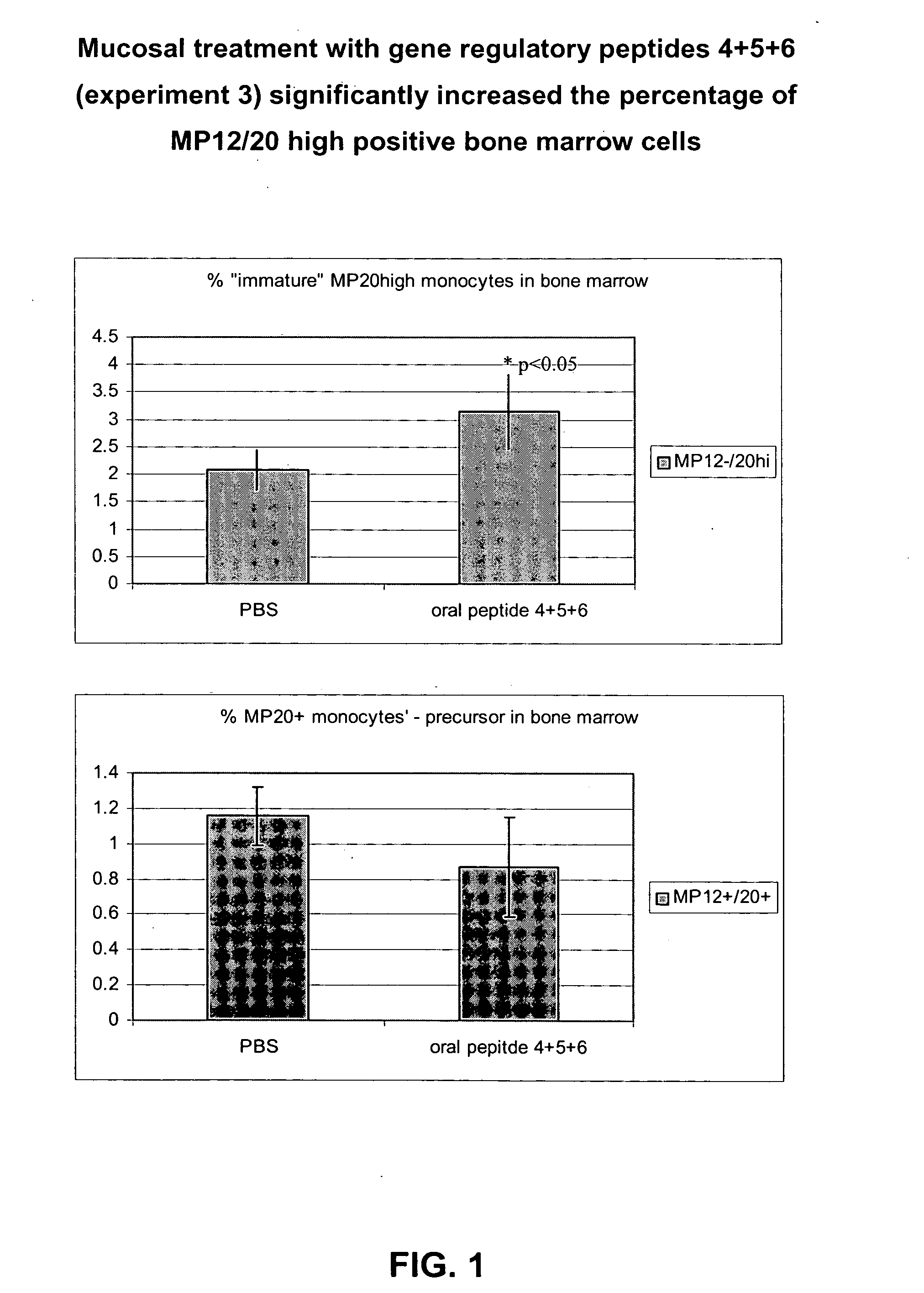
Compositions for mucosal and oral administration comprising hcg fragments
a technology of mucosal and oral administration, applied in the field of biotechnology, can solve the problems of life-threatening systemic disease, toxic when secreted into the circulation, and a host's deleterious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0037]Not wishing to be bound by theory, it is postulated herein that an unexpected mode of gene regulation with far reaching consequences for the oral or mucosal treatment of disease has been uncovered. Polypeptides, such as endogenous CG, EGF, etc., but also polypeptides of pathogens such as viral, bacterial or protozoal polypeptides, are subject to breakdown into distinct oligopeptides, for example, by intracellular proteolysis. Distinct proteolytic enzymes are widely available in the cell, for example, in eukaryotes in the lysosomal or proteasomal system. Some of the resulting breakdown products are oligopeptides of 3 to 15, preferably 4 to 9, most preferably 4 to 6, amino acids long that are surprisingly not without any function or effect to the cell, but as demonstrated herein may be involved, possibly via a feedback mechanism in the case of breakdown of endogenous polypeptides, as signaling molecules in the regulation of gene expression, as demonstrated herein by the regulati...
example
[0136]This invention in particular relates to the, preferably oral, treatment of multiple sclerosis, and in particular to the, preferably oral, treatment of the inflammatory injury seen in the progressive stages in the disease such as seen with the recurrent upsurges of acute disease, classically known as relapses or exacerbations, herein identified as relapsing / remitting disease seen with multiple sclerosis (MS).
[0137]Multiple sclerosis (MS) is the prototype inflammatory autoimmune disorder of the central nervous system and, with a lifetime risk of one in 400, potentially the most common cause of neurological disability in young adults. In experimental animals, an experimental autoimmune / allergic encephalomyelitis (EAE) can be induced in which MS is studied. Exacerbations in EAE and MS both are dramatically mediated by cytokines and chemokines. During an exacerbation, the TNF-α family and other pro-inflammatory cytokines is highly elevated in CSF. As with all complex traits, the di...
experiment 1
[0159]Material and methods: Female NOD mice were bred and maintained in a pathogen-free facility at Lucky Farm, Balkbrug, The Netherlands. All mice were given free access to food and water.
[0160]Twenty-one to 22-week-old diabetic female NOD mice (n=5) were given four weeks long free access to either water containing 4 IU per ml hCG (pregnyl; batch number 235863) or mixture of gene-regulatory peptides LQGV (SEQ ID NO:1), GVLPALPQ (SEQ ID NO:23) and VLPALP (SEQ ID NO:4) (each 1 microgram per milliliter). Control mice were given plain water only. During these four weeks of treatment mice were daily observed for their drinking behavior, urination, and the look of the fur.
[0161]Results: During four weeks of treatment, mice without treatment drank much water because on daily bases their drinking bottle had to be refilled and they had percolated fur, which is a normal sign of heavily diabetic mice. Mice with treated water with hCG or a mixture of gene-regulatory peptides drank a normal amo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com