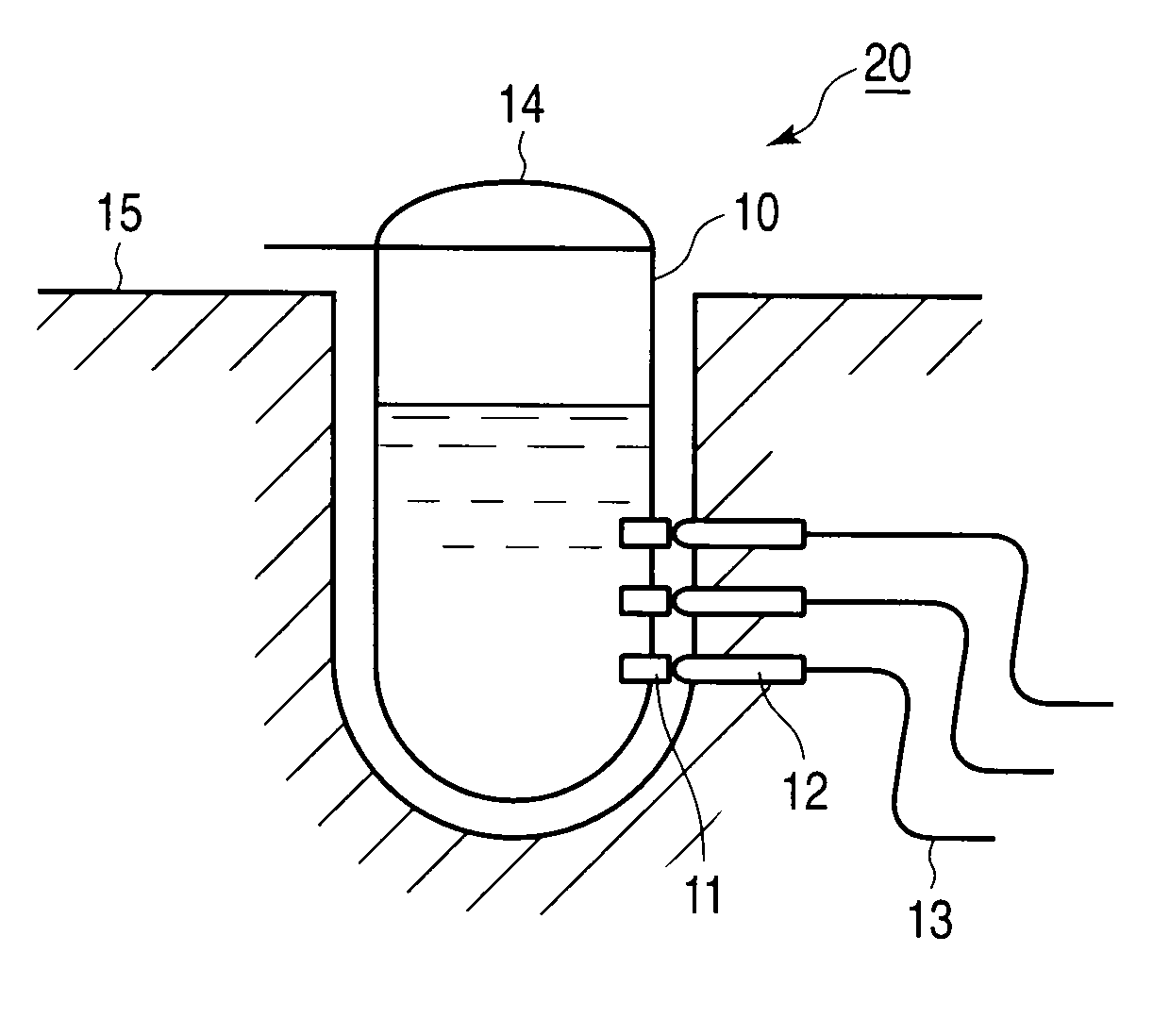
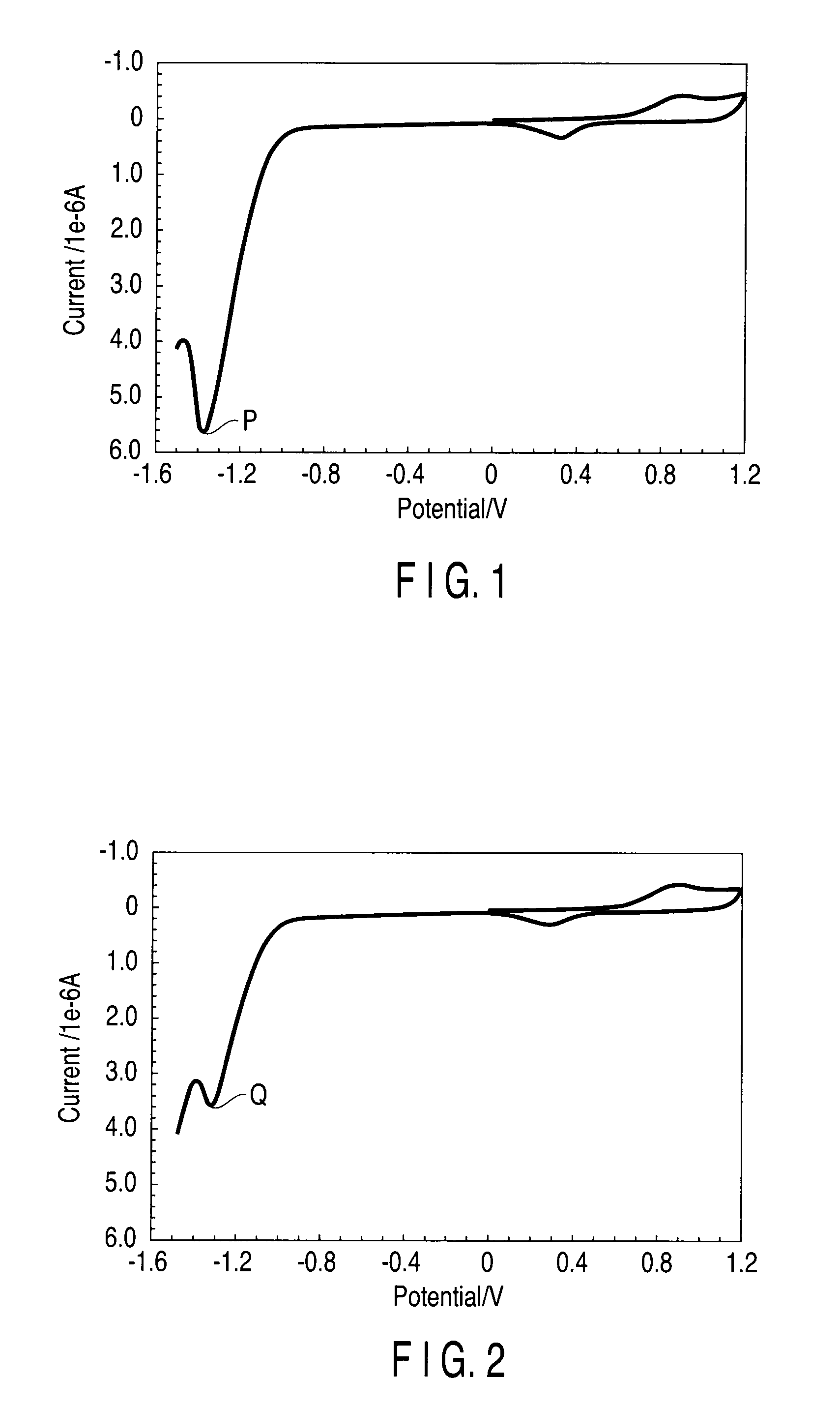
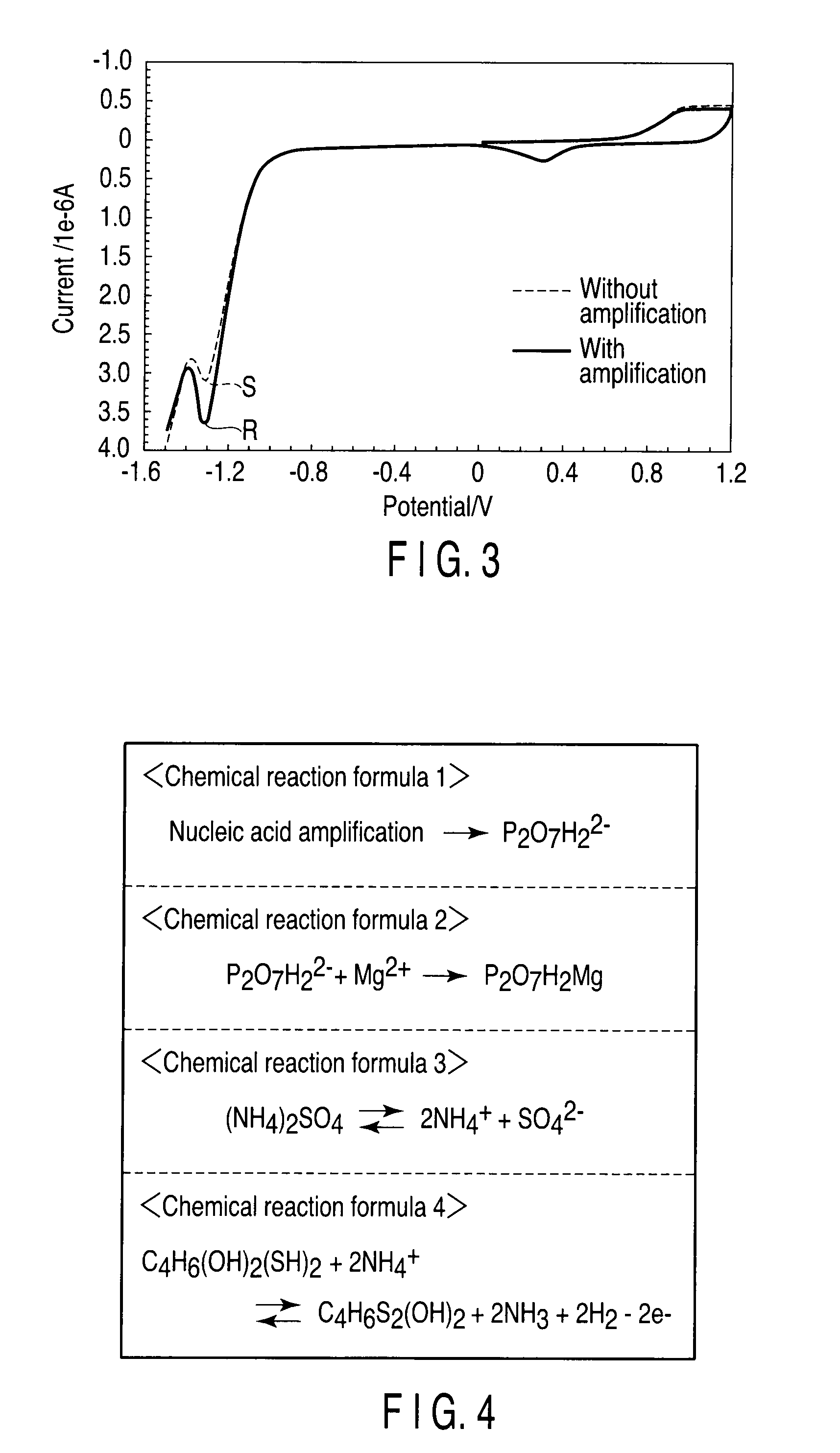
Method of detecting nucleic acid amplification reaction
a nucleic acid and amplification technology, applied in the field of nucleic acid amplification reaction detection methods, can solve the problems of complex detection process, difficult accurate measurement, poor oxidation resistance of nucleic acid, etc., and achieve the effect of simple structure, accurate measurement and simple measuremen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
(1) Preparation of Nucleic Acid Amplification Buffer
[0025]First, a nucleic acid amplification buffer is prepared. This buffer is an amplification buffer which contains not only a polymerase, a primer and an NTP substrate, but also a reducing agent molecule, a redox substance, and a magnesium ion. The former 3 substances are substances for amplifying a nucleic acid, and the latter 3 substances are substances for stabilizing a nucleic acid during amplification reaction. In addition, arbitrary components are contained appropriately in the buffer.
[0026]The amplification reaction in the first embodiment is not limited as long as it is an amplification reaction using a polymerase and NTP substrate, and this amplification reaction is selected for example from the group consisting of PCR, LAMP, ICAN and SMAP. Accordingly, the buffer contains a polymerase adapted to the amplification reaction to be carried out. For example, when the amplification reaction is conducted by PCR, a thermostable ...
example 1
1. Materials Used, Etc.
[0064](1) Nucleic Acid Amplification Buffer
[0065]In the nucleic acid amplification buffer, a primer set for model gene A amplification shown below, an enzyme for LAMP amplification, and a buffer were used. As a template, sample nucleic acid V of unknown sequence was used.
[0066]Model Gene A: Mouse 2C39
[0067]Primer Set for Model Gene A Amplification
TCAAAACGATCCTGGAAAATAATGGACATTCATTCTGAGCTGTGCGGAAAAACTAAATGAGAATGTCAAGCAGAAAAAACATTCTTGACTTCTTCACGCTCACCTTGTGACTGTGGCAATAAAGCACCAGCAGATGACATTGCATGGA
[0068](2) Electrodes
[0069]A working electrode, a counter electrode and a reference electrode used as electrochemical measurement electrodes were gold electrodes.
2. Experimental Procedures
[0070]First, an amplification reaction solution was prepared. The primer set for model gene A amplification, the enzyme for LAMP amplification and the buffer were mixed, and the sample nucleic acid V was added as a template.
[0071]Then, electrochemical measurement was conducted before the i...
example 2
1. Materials Used, Etc.
[0075](1) Nucleic Acid Amplification Buffer
[0076]In the nucleic acid amplification buffer, primer sets for model genes A and B amplification shown below, an enzyme for LAMP amplification, and a buffer were used. As templates, sample nucleic acids X and Y of unknown sequence were used.
[0077]Model Gene A: Mouse 2C39
[0078]Primer Set for Model Gene A Amplification
TCAAAACGATCCTGGAAAATAATGGACATTCATTCTGAGCTGTGCGGAAAAACTAAATGAGAATGTCAAGGAGAAAAAACATTCTTGACTTCTTCAGGCTCACCTTGTGACTGTGGCAATAAAGCACCAGCAGATGACATTGCATGGA
[0079]Model Gene B: Mouse 481
[0080]Primer Set for Model Gene B Amplification
ATTTGGAACATACTGCTCTCTTCTGCTGCCATCTTCCTTTTGACAAACTCAGACCTCCTTGAAAAGAACACAAAATCCTCGATAACTCGGATCTGGGAAGGATCAGCCTGTCTGAAGATAGCTATTCACA
[0081](2) Electrodes
[0082]A working electrode, a counter electrode and a reference electrode used as electrochemical measurement electrodes were gold electrodes.
2. Experimental Procedures
[0083]First, amplification reaction solutions were prepared. In amplifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com