Method and device for activating stem cells
a stem cell and activation method technology, applied in the field of stem cell activation devices and methods, can solve problems such as unintended side effects, and achieve the effects of enhancing an osteoblast phenotyp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0098]The following materials and methods were used to develop certain aspects of the invention.
Differentiation of Cells
[0099]Human mesenchymal stem cells (Lonza, Walkersville, Md.) at passage 3 were seeded in basal medium (Stem Cell Technologies, Vancouver, Canada) at a density of 6×104 cells / 35 mm well and incubated for 2 days at 37° C. Cells were rinsed with PBS, activated with 100 ng / ml of growth factor (FGF-2 or TGFβ3, R&D Systems, Minneapolis, Minn.) in fresh basal medium for 1 hour, rinsed with PBS and then incubated in either fresh basal medium or osteogenic differentiation medium (Stem Cell Technologies, Vancouver, Canada) for 7-14 days at 37° C.
Real time PCR
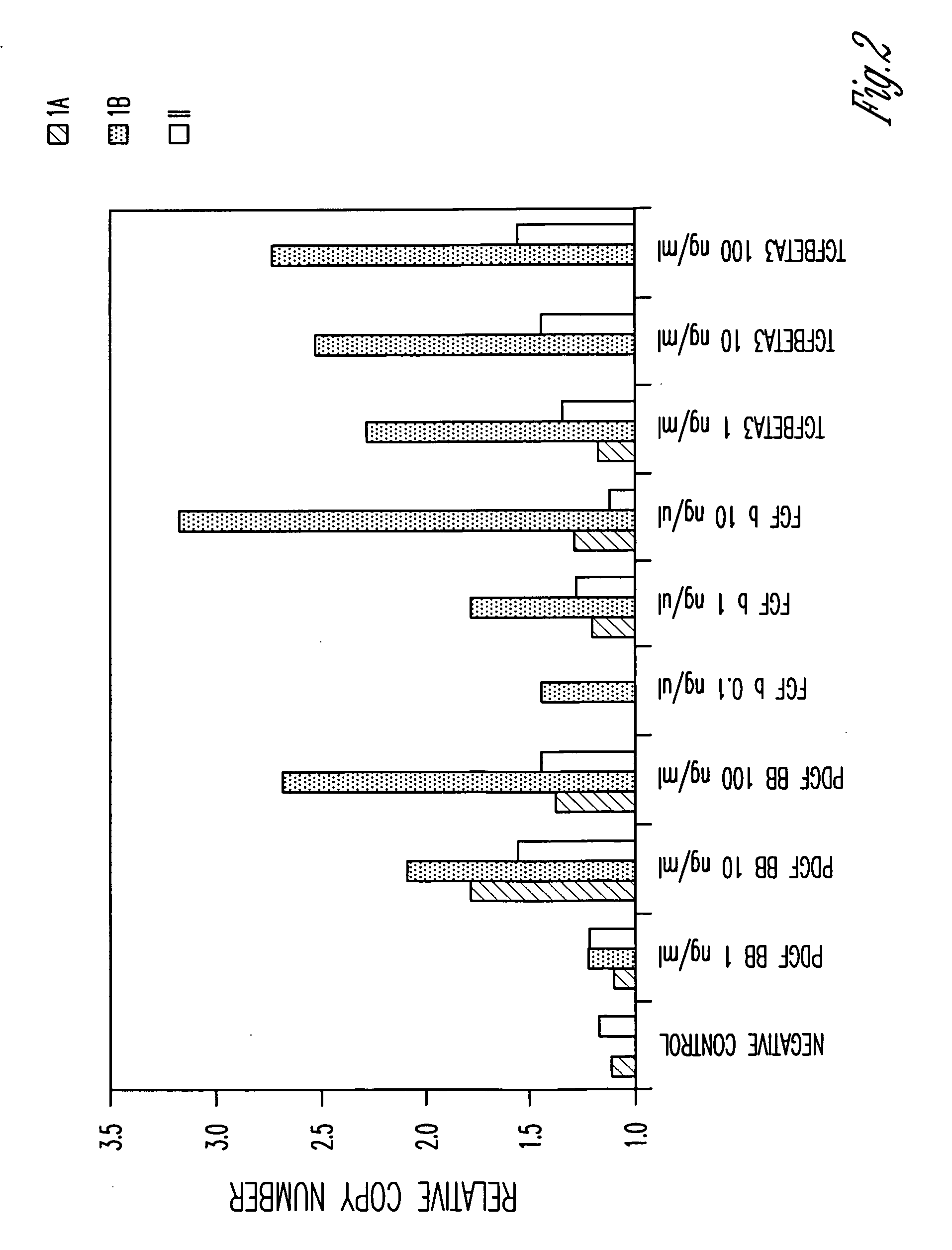
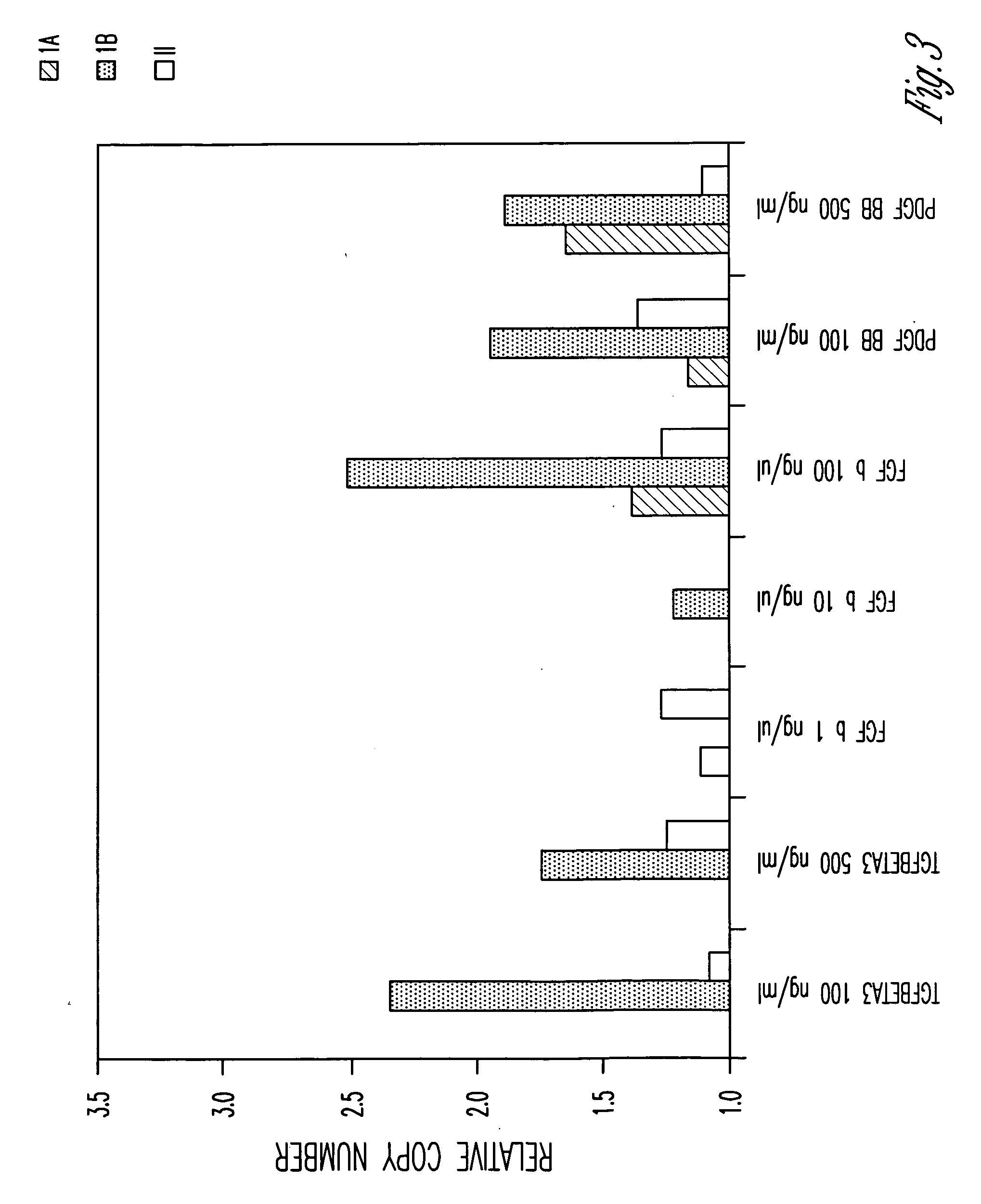
[0100]Total RNA was prepared from cells treated with various active agents using RNeasy Plus Mini Kit and QIA shredder Mini Spin columns (Qiagen). Total RNA was also prepared from untreated cells as a control. cDNA was generated using random hexamer and Oligo dT following the TaqMan Reverse Transcri...
example 2
Results
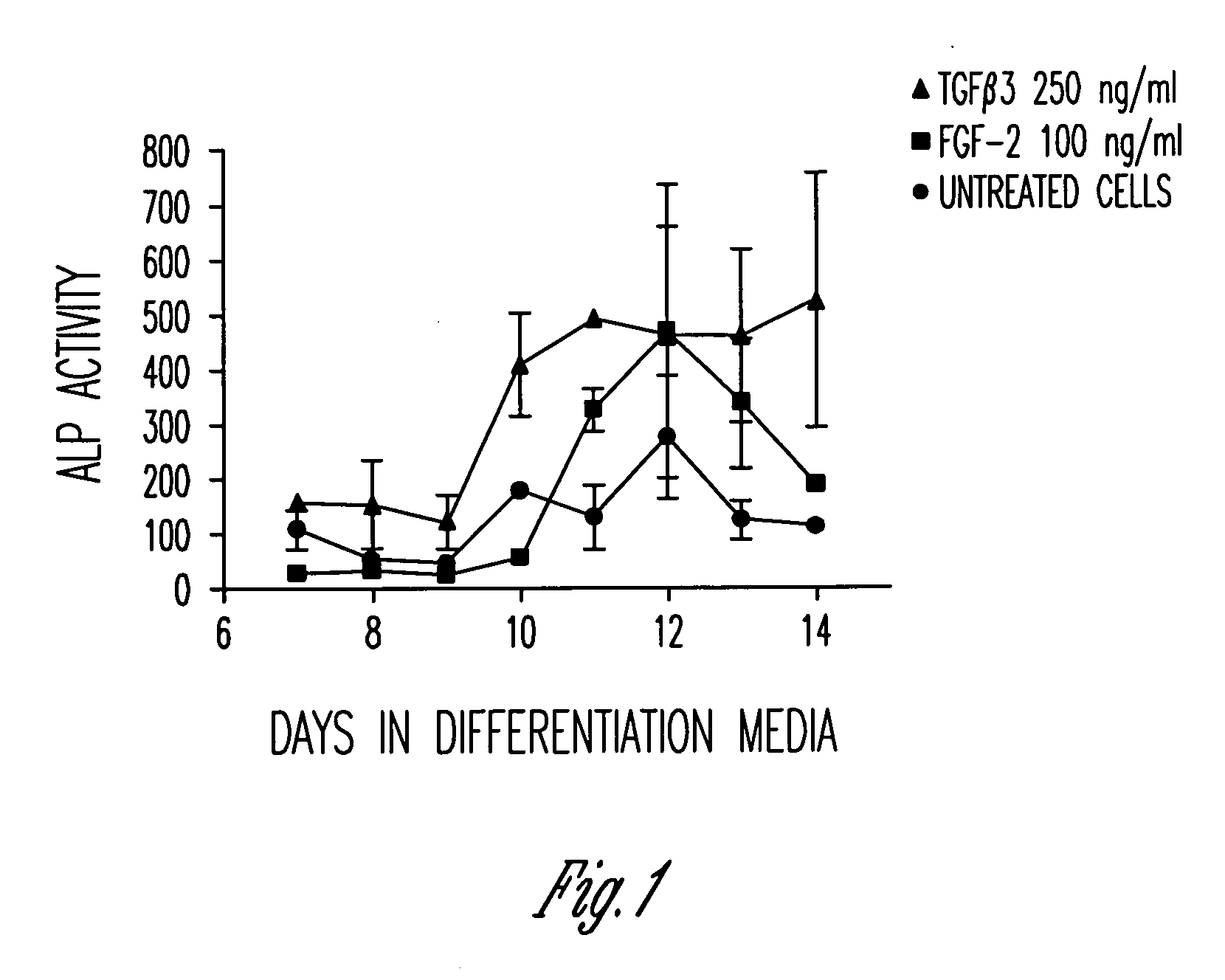
[0106]Primary human mesenchymal stem cells are capable of differentiating down an osteoblastic lineage. This is demonstrated in vitro by culturing cells in an osteogenic cocktail containing, but not limited to, dexamethasone, ascorbic acid and β-glycerophosphate. Under these culture conditions cells show an upregulation of osteoblast differentiation markers, the most common of which is the early marker alkaline phosphatase.
[0107]FIG. 1 shows a slight upregulation in alkaline phosphatase activity in the untreated mesenchymal stem cells (circles) from days 10 to 12, as expected. However, when the mesenchymal stem cells were pretreated with TGFβ3 (triangles) or FGF-2 (squares) for 1 hour, followed by removal of the agents and culture in differentiation media, the level of alkaline phosphatase activity increased significantly 2-3 fold.
[0108]These data indicate that just a 1 hour treatment of mesenchymal stem cells with either TGFβ3 or FGF-2 on day 0 can impact osteoblast differen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com