Intratumorally administered lactoferrin in the treatment of malignantneoplasms and other hyperproliferative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
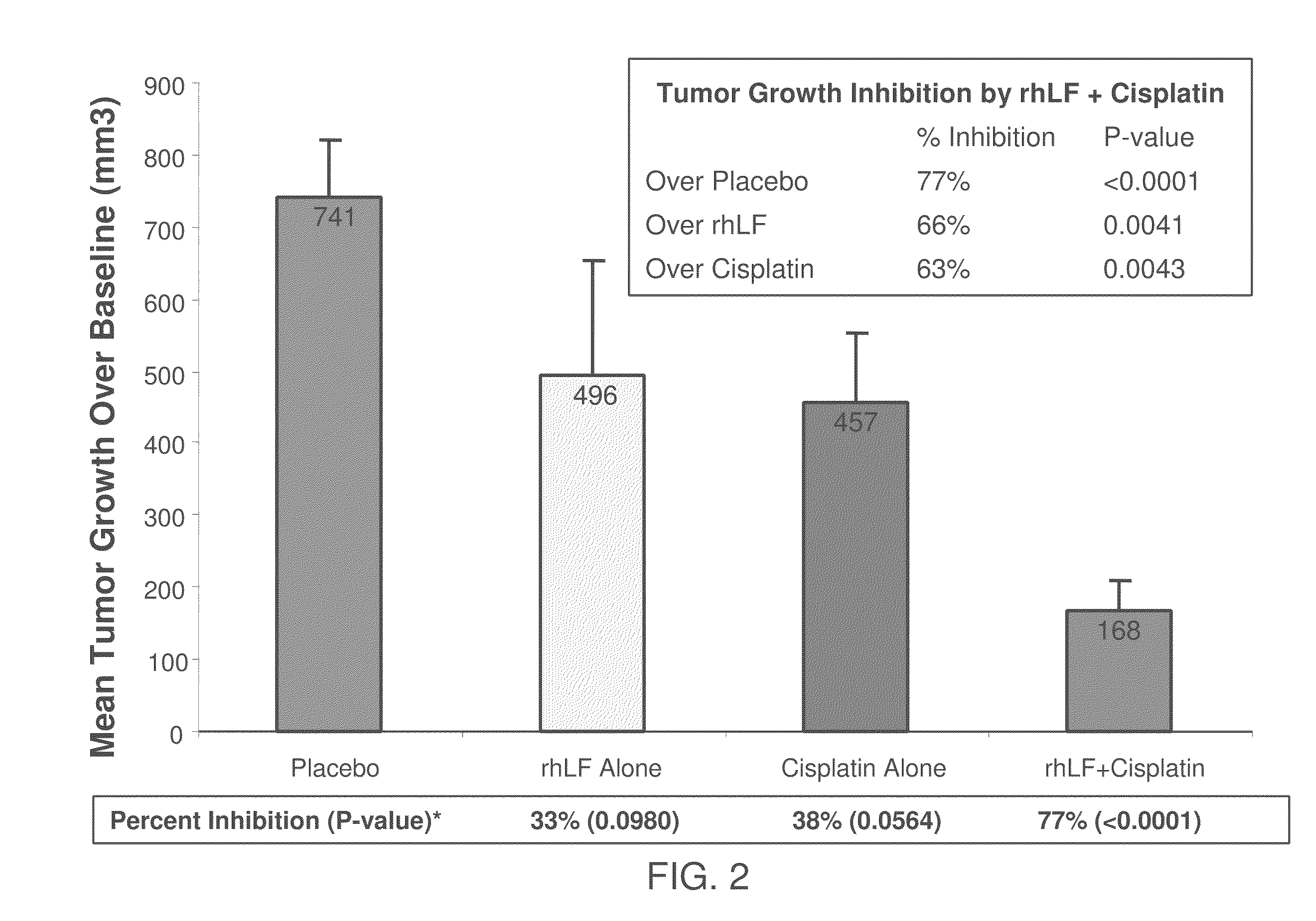
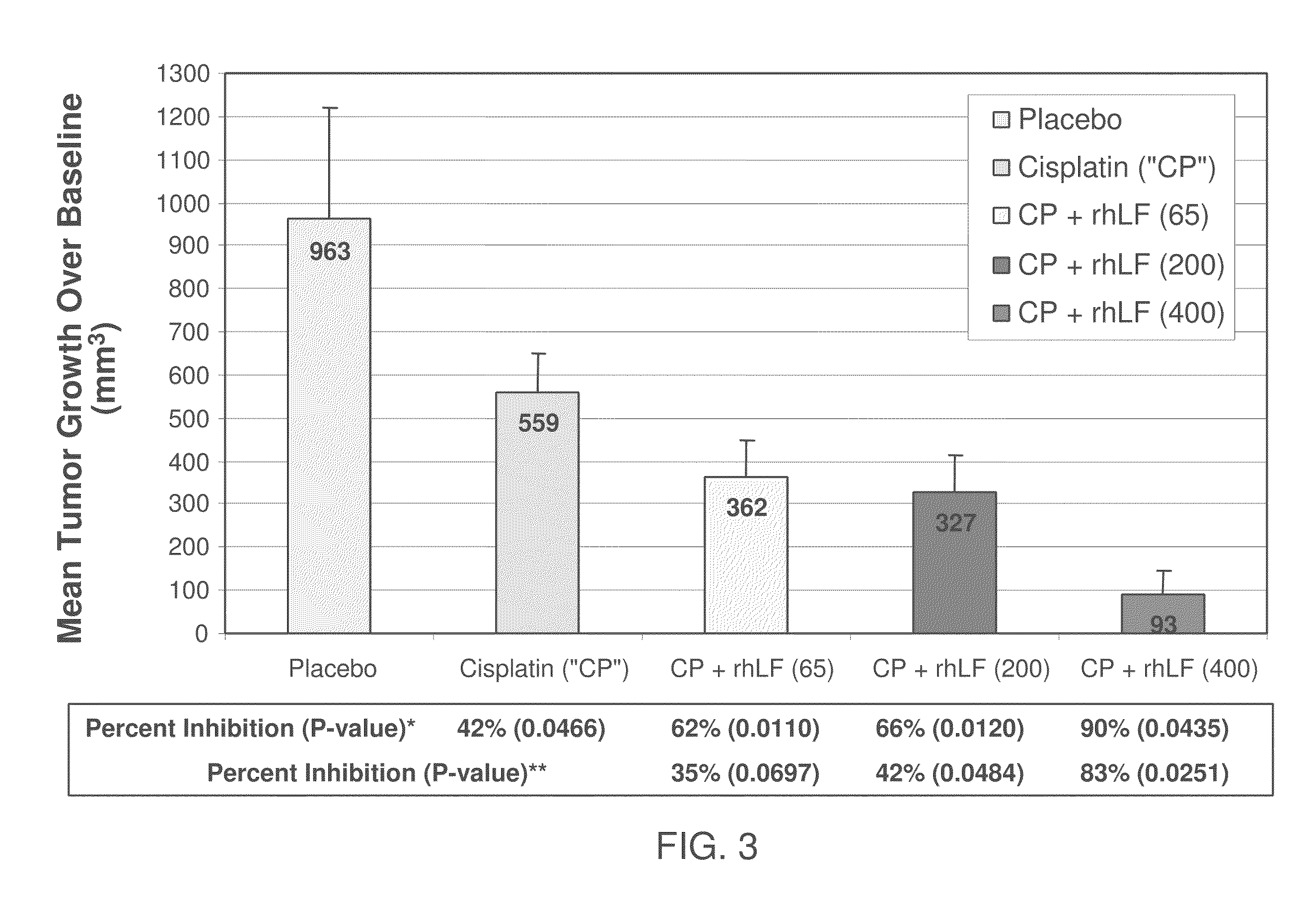
Inhibition of Tumor Growth by rhLF
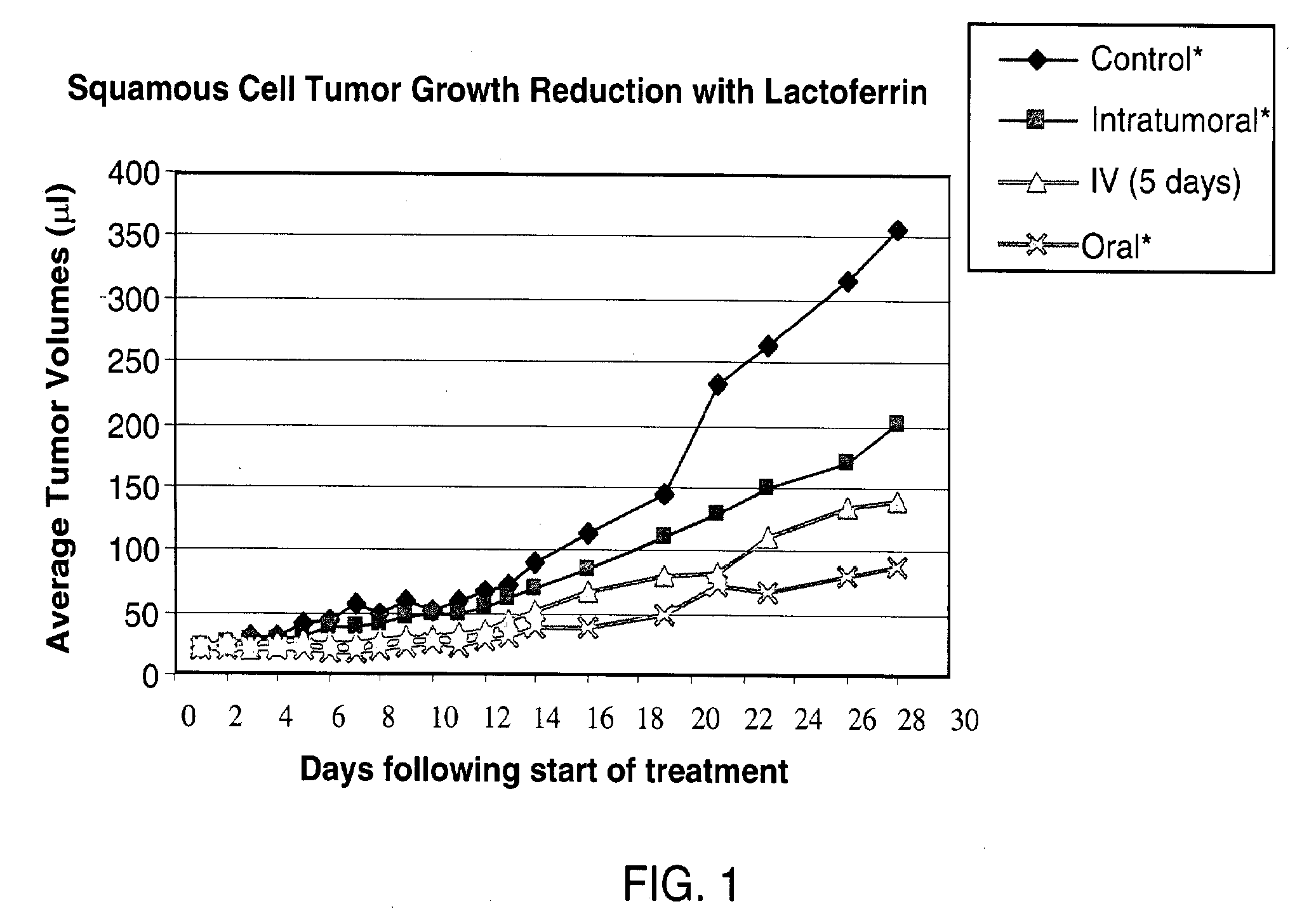
[0088]Human squamous cell carcinoma (O12) was used. The cells were injected into the right flank of athymic nude mice. rhLF was administered either intratumorally (49 animals, 7 doses ranging from 0.05 μg to 125 μg per dose), intravenously (7 animals, 125 μg / dose) or orally (7 animals 20 mg / dose). Control animals were treated with only the vehicle; no rhLF was administered to the control animals. rhLF was administered twice a day for either five days (intravenous group) or eight days (all other groups) starting 11 days after inoculation with tumor cells to allow formation of established tumors.
[0089]The efficacy of treatment was evaluated by measuring the solid tumor size during and at the end of the experiment; the body weights were also determined at the time of tumor measurements. As seen in FIG. 1 and Table 1, treatment with rhLF reduced rates of tumor growth relative to the control by 46% to 80%. In fact, oral treatment with 20 mg rhLF most sig...
example 2
Evaluation of rhLF in Tumor Types
[0091]Tumor cells from a broad range of tumor types are injected into the right flank of athymic nude mice. Animals are administered either rhLF, native hLF or bovine LF orally. Control animals are treated with only the vehicle, no rhLF is administered to the control animals. rhLF is administered either once or twice a day for either one, five, seven or fourteen days or eight days starting approximately eleven days after inoculation with tumor cells to allow formation of established tumors or at such other time as is generally done with standard or published regimens.
[0092]The efficacy of treatment is evaluated by measuring the solid tumor size during and at the end of the experiment; the body weights are also determined at the time of tumor measurements. The immune response is measured by measuring the amount of cytokines, T-cells and NK cells in circulation and in the intestine.
example 3
Effect of Oral Administration of rhLF and bLF
[0093]Recombinant human lactoferrin and bovine lactoferrin were orally administered to mice, and the production of IL-18 in the small intestine was measured.
[0094]Mice were treated for three days daily with 65 mg / kg / day of rhLF, 300 mg / kg / day of rhLF or 300 mg / kg / day of bLF. For a control, mice were only administered the pharmaceutical carrier. Twenty-four hours following administration of the LF or control for 3 days, animals were weighed and blood and serum were collected. Serum was used for cytokine ELISA assays.
[0095]Also, at these time points, animals were sacrificed and the small intestinal tissue was removed for further analysis. Small intestinal epithelium was homogenized using a lysis buffer consisting of PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulphate containing 10 μg / ml PhenylMetheylsulfonyl fluoride. Homogenate was centrifuged at 15,000 rpm for 10 minutes and the supernatant stored at −80 C til...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass flow rate | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com