Noscapine analogs and their use in treating cancers, including drug-resistant cancers
a technology of nascapine and its analogs, which is applied in the field of nascapine analogs, can solve the problems of hypersensitivity reactions, limited pharmacological profile of microtubule-binding agents, and limited use of microtubule drugs such as vincas and taxanes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
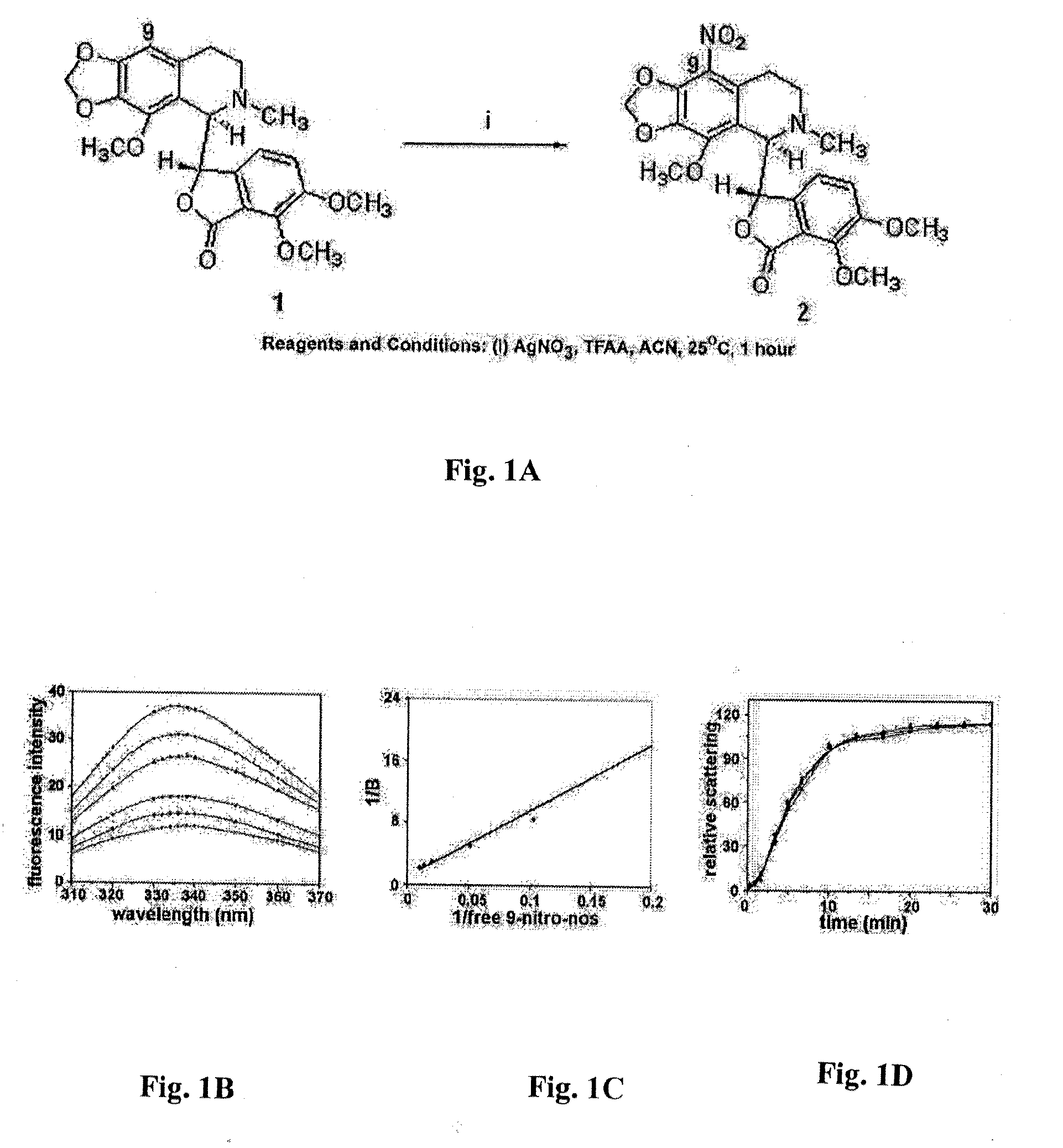
Preparation of 9-Nitro-Nos((S)-6,7-dimethoxy-3-((R)-4-methoxy-6-methyl-9-nitro-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)isobenzofuran-1(3H)-one)
[0086]To a solution of noscapine (4.134 g, 10 mmol) in acetonitrile (50 ml), silver nitrate (1.70 g, 10 mmol) and trifluoroacetic anhydride (5 ml, 35 mmol) were added. After one hour of reaction time, the reaction progress was monitored using thin layer chromatography (10% methanol in chloroform) and the reaction mixture was poured into 50 ml of water and extracted with chloroform (3×50 ml). The organic layer was washed with brine, dried over anhydrous MgSO4 and the solvent was evaporated in vacuo. The desired product, (S)-6,7-dimethoxy-3-((R)-4-methoxy-6-methyl-9-nitro-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinolin-5-yl)isobenzofuran-1(3H)-one (9-nitro-nos) was obtained as yellow crystalline powder by flash chromatography (silica gel, 230-400 mesh) with 10% methanol in chloroform as an eluent. mp 178.2-178.4° C.; IR: 1529, ...
example 2
Evaluation of the Tumulin Binding Properties of 9-Nitro-Nos
[0093]Cell Lines and Chemicals:
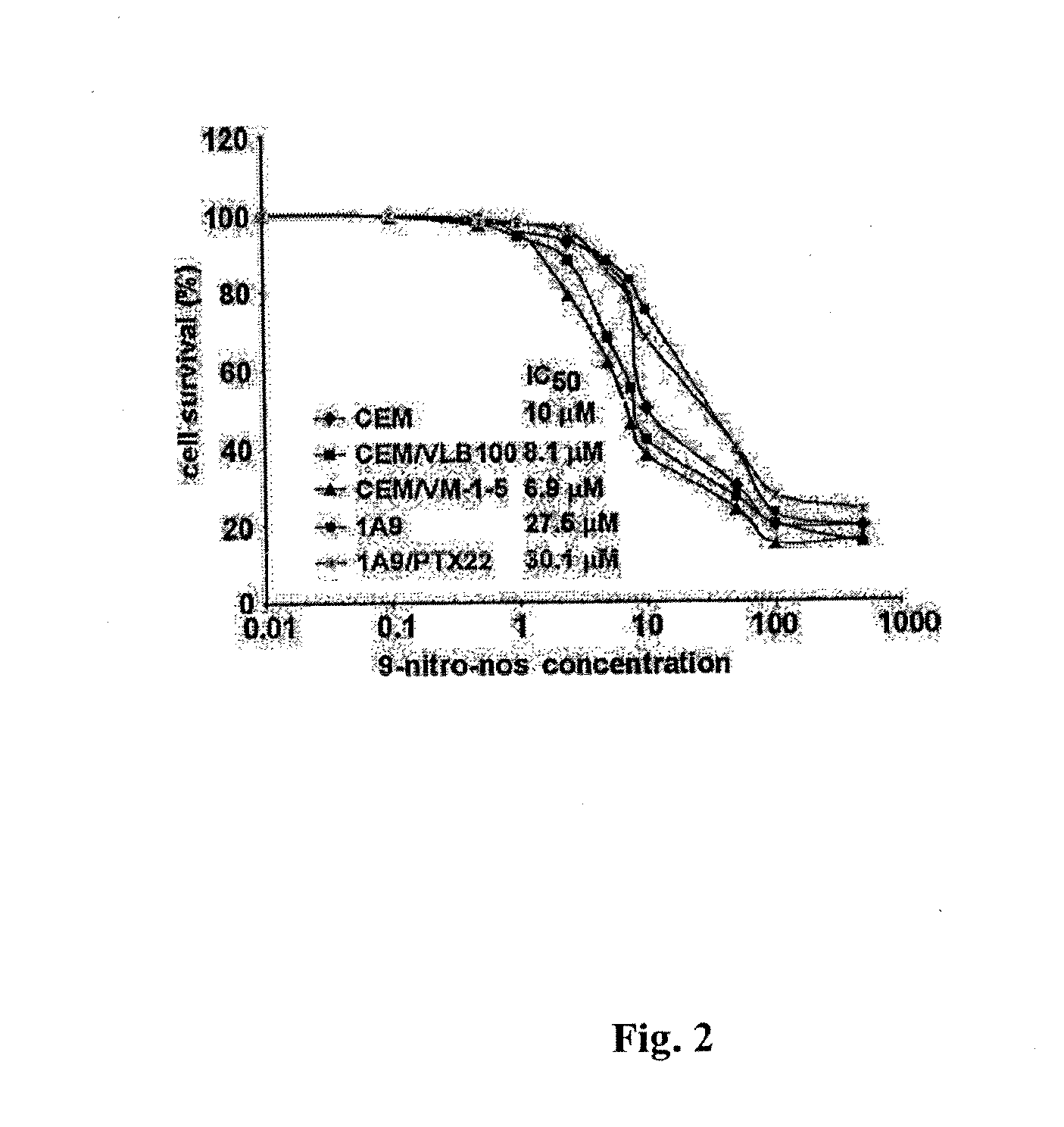
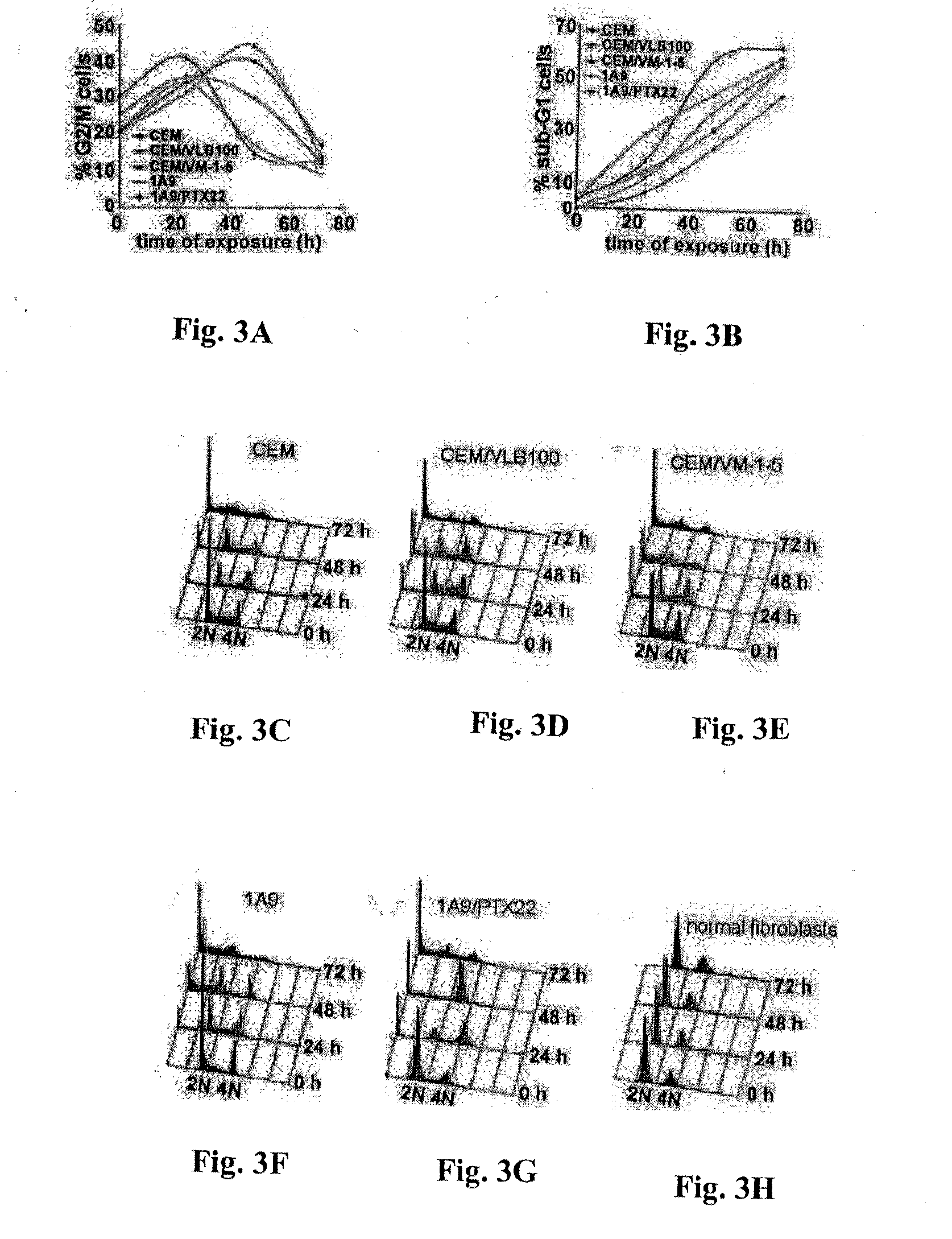
[0094]Cell culture reagents were obtained from Mediatech, Cellgro. CEM, a human lymphoblastoid line, and its drug-resistant variants—CEM / VLB100 and CEM / VM-1-5, were provided by Dr. William T. Beck (Cancer Center, University of Illinois at Chicago). CEM-VLB100, a multi-drug resistant line selected against vinblastine is derived from the human lymphoblastoid line, CEM and expresses high levels of 170-kd P-glycoprotein (Beck and Cirtain, 1982). CEM / VM-1-5, resistant to the epipodophyllotoxin, teniposide (VM-26), expresses a much higher amount of MRP protein than CEM cells (Morgan et al., 2000). The 1A9 cell line is a clone of the human ovarian carcinoma cell line, A2780. The paclitaxel-resistant cell line, 1A9 / PTX22, was isolated as an individual clone in a single-step selection, by exposing 1A9 cells to 5 ng / ml paclitaxel in the presence of 5 μg / ml verapamil, a P-glycoprotein antagonist (Giannaka...
example 3
Synthesis of Halogenated Noscapine Analogues
(S)-3-((R)-9-bromo-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquino-lin-5-yl)-6,7-dimethoxyisobenzofuran-1(3H)-one
[0169]To a flask containing noscapine (20 g, 48.4 mmol) was added minimum amount of 48% hydrobromic acid solution (˜40 ml) to dissolve or make a suspension of the reactant. To the reaction mixture was added freshly prepared bromine water (˜250 ml) drop wise until an orange precipitate appeared. The reaction mixture was then stirred at room temperature for 1 h to attain completion, adjusted to pH 10 using ammonia solution to afford solid precipitate. The solid precipitate was recrystallized with ethanol to afford bromo-substituted noscapine. Yield: 82%; mp 169-170° C.; IR: 2945 (m), 2800 (m), 1759 (s), 1612 (m), 1500 (s), 1443 (s), 1263 (s), 1091 (s), 933 (w) cm−1; 1H NMR (CDCl3, 400 MHz), δ 7.04 (d, 1H, J=7 Hz), 6.32 (d, 1H, J=7 Hz), 6.03 (s, 2H), 5.51 (d, 1H, J=4 Hz), 4.55 (d, 1H, J=4 Hz), 4.10 (s, 3H), 3.98 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com