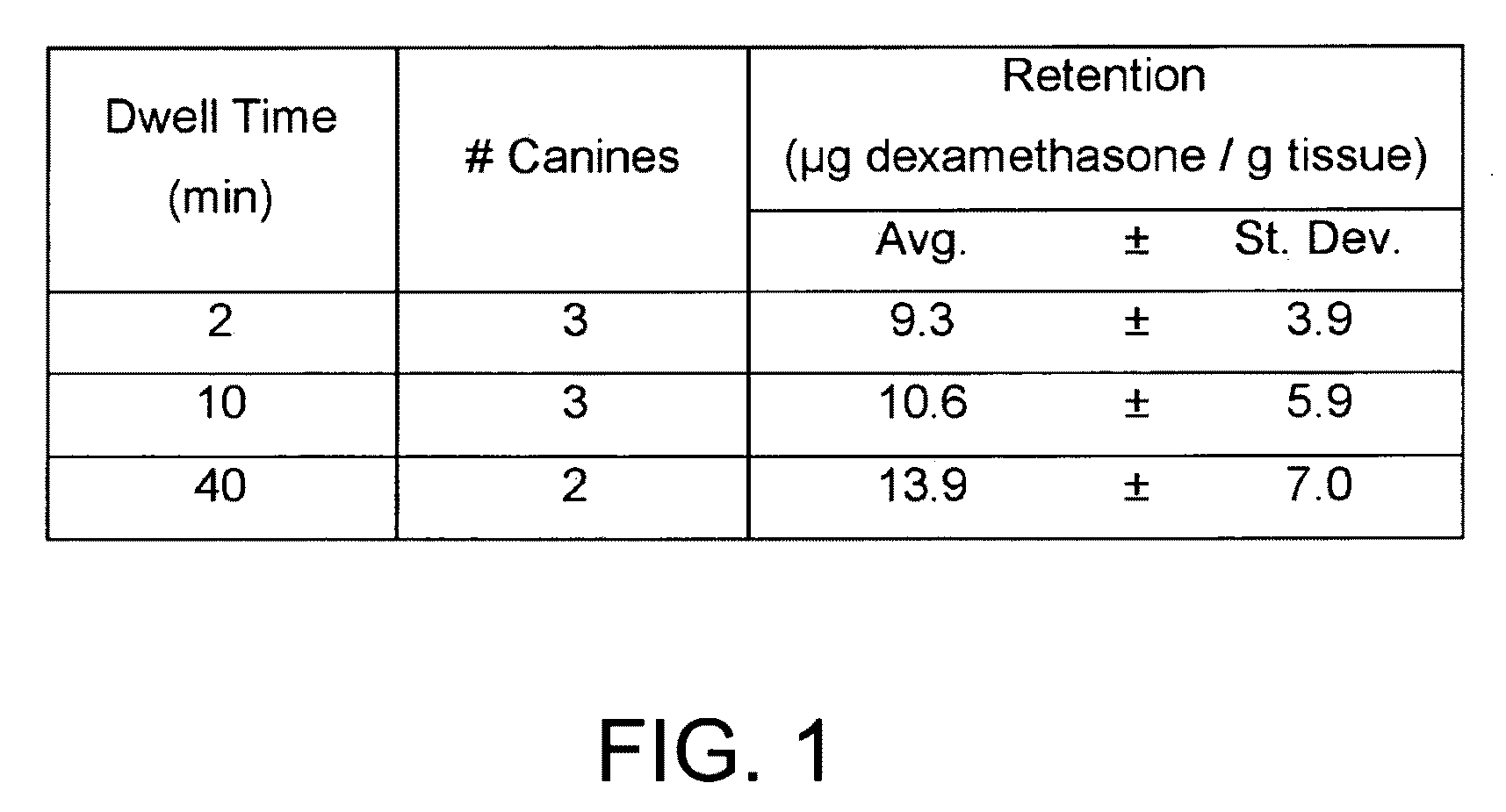
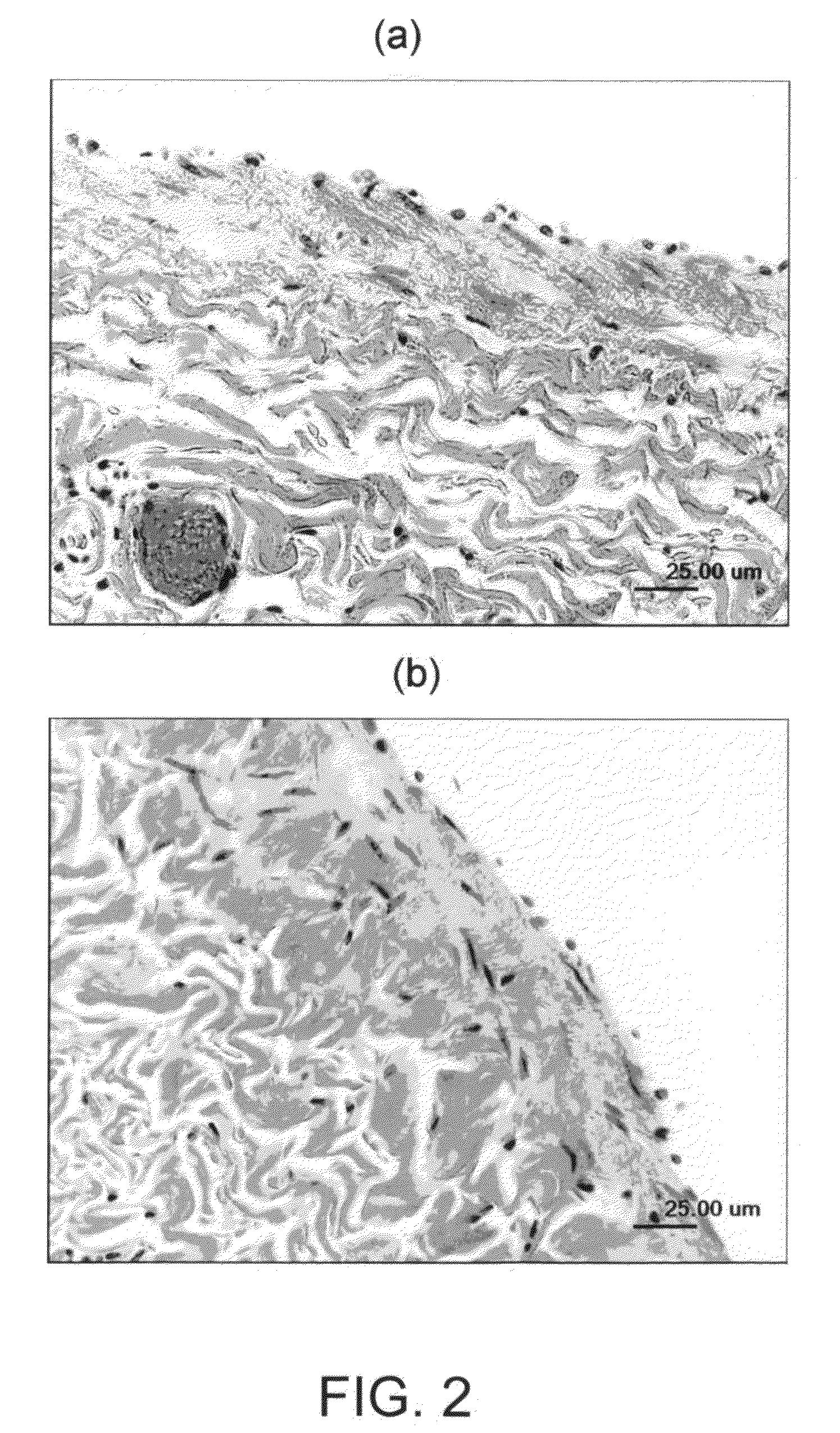
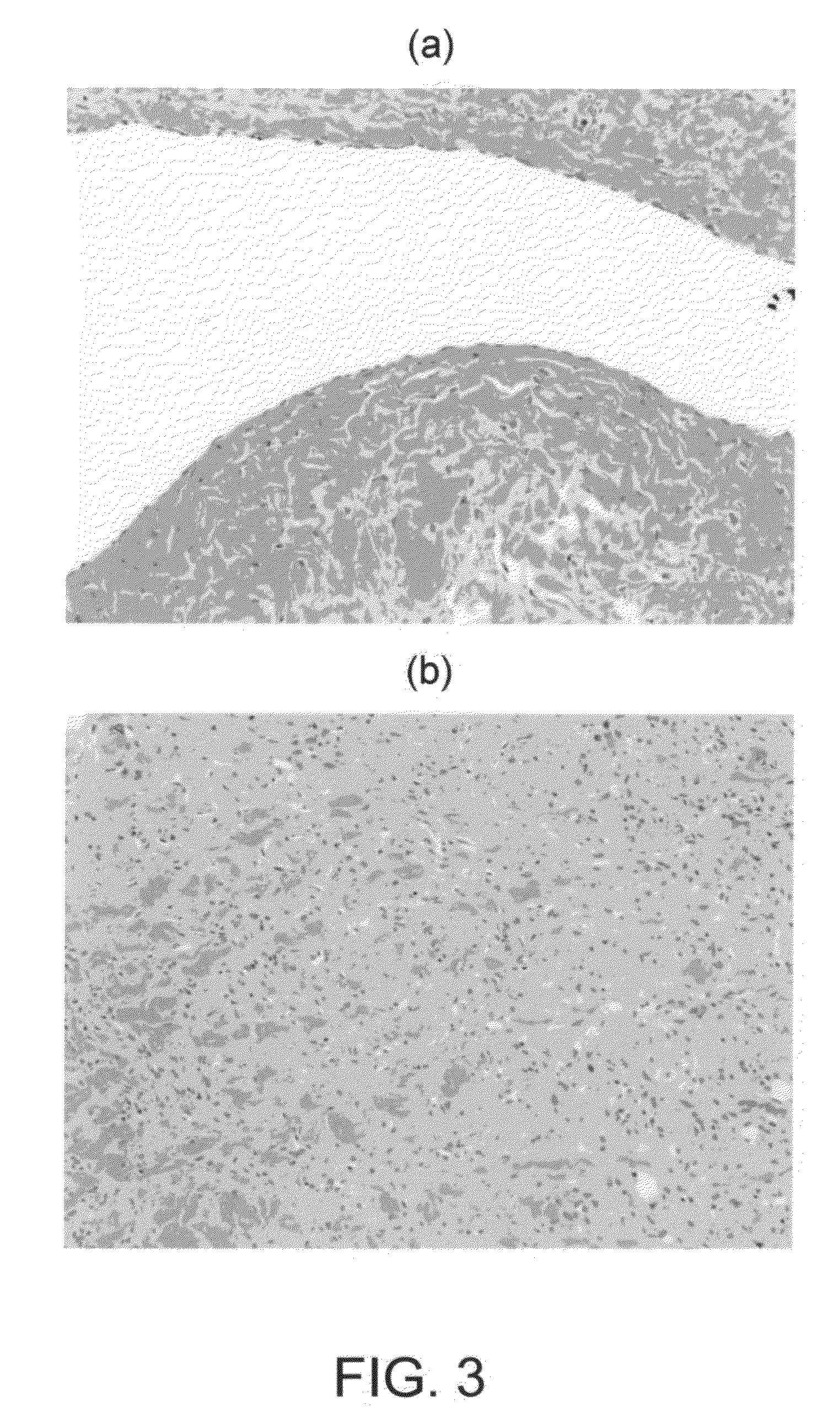
Articles and methods of treating vascular conditions
a technology of vascular conditions and articles, applied in the field of articles and methods of treating vascular conditions, can solve the problems of mechanical trauma to insufficient delivery and retention of bioactive agents at the vascular treatment site using this approach, and long dwell time at the vascular treatment si
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070]This Example describes the preparation of a thixotropic, turbid gel material that contains a bioactive agent capable of treating vascular tissue in sufficient amounts to treat a vascular condition.
[0071]A first solution (referred herein as Solution 1A) was prepared by mixing phosphate buffered saline (PBS) (0.15M NaCl, pH 7.4, Invitrogen Corporation Carlsbad, Calif.) with 0.40 g / ml hydroxypropyl-β-cyclodextrin (HPβCD) (Sigma-Aldrich, St. Louis, Mo.) and 0.20 g / ml alpha-cyclodextrin (αCD) (Sigma-Aldrich) through stirring and heating (60° C.), followed by adding dexamethasone (Pharmacia & Upjohn Company, Kalamazoo, Mich.) at 20 mg / ml with stirring and heating (60° C.). Solution 1A did not form a gel material and was not turbid.
[0072]A second solution (referred herein as Solution 1B) was prepared by dissolving polyethylene glycol (PEG, Dow Chemical, Midland, Mich.) of average Mn=8 kDa (0.26 g / ml) with PBS. Solution 1B did not form a gel material and was not turbid.
[0073]Equal vol...
example 2
[0074]This Example describes preparation of a thixotropic, turbid gel material that contains a bioactive agent capable of treating vascular tissue in sufficient amounts to treat a vascular condition.
[0075]A first solution (Solution 2A) was prepared by mixing PBS (0.15M NaCl, pH 7.4, Invitrogen) with 0.40 g / ml hydroxypropyl-β-cyclodextrin (HPβCD) (Sigma-Aldrich, St. Louis, Mo.) and 0.20 g / ml alpha-cyclodextrin (αCD) (Sigma-Aldrich) through stirring and heating (60° C.), followed by adding 17β-estradiol (20 mg / ml) (Sigma-Aldrich) by stirring and heating (60° C.). Solution 2A did not form a gel material and was not turbid.
[0076]A second solution (Solution 2B) was prepared by dissolving PEG (Dow Chemical, Midland, Mich.) of average Mn=8 kDa (0.26 g / ml) in PBS. Solution 2B did not form a gel material and was not turbid.
[0077]Equal volumes of Solution 2A and Solution 2B were combined with mixing to form Gel Material B. Gel Material B was turbid, and was opaque and white in appearance.
example 3
[0078]This Example describes preparation of a thixotropic, turbid gel material that contains a bioactive agent capable of treating vascular tissue in sufficient amounts to treat a vascular condition.
[0079]A first solution (Solution 3A) was prepared by mixing PBS (0.15M NaCl, pH 7.4) with 0.40 g / ml hydroxypropyl-β-cyclodextrin (HPβCD) (Sigma-Aldrich, St. Louis, Mo.) and 0.20 g / ml alpha-cyclodextrin (αCD) (Sigma-Aldrich) through stirring and heating (60° C.), followed by adding dicumarol (0.67 mg / ml) (Sigma-Aldrich) by stirring and heating (60° C.). Solution 3A did not form a gel material and was not turbid.
[0080]A second solution (Solution 3B) was prepared by dissolving of PEG (Dow) of average Mn=8 kDa (0.26 g / ml) in PBS. Solution 3B did not form a gel material and was not turbid.
[0081]Equal volumes of solutions 3A and 3B were combined with mixing to form Gel Material C. Gel Material C was turbid, and was opaque and white in appearance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com