Composition and method for measuring thallium influx and efflux
a technology of thallium influx and efflux, applied in the field of methods for detecting the activity of an ion channel in a cell, can solve the problems of limited hts screening (e.g., hcs) of ion channels, and current methods, however, have significant drawbacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0133]The following is in example of the components that may be used in some of the methods of the present invention.
[0134]Component A:
Per Vial:FluoZin-1 AM ester (Invitrogen) 1-20 μgLyophilized and stored in a single use 15 mL vial
[0135]Component B: 5× Assay Buffer: 1×25 mL
Per Vial:50% Vol 10X HBSS12.5 mL10% Vol 1 M HEPES 2.5 mL40% Vol H2O 10 mL
[0136]Component C: 5× Chloride Free Stimulus Buffer: 1×25 mL
Per vial: 700 mM Na Gluconate3.82 g12.5 mM K Gluconate 73 mg 9 mM Ca Gluconate96.7 mg 5 mM Mg Gluconate51.7 mg 100 mM HEPES 2.5 mL 1MWater22.5 mL
[0137]Component D: Concentrated (125 mM) K2SO4 Solution: 1×20 mL
Per vial125 mM Potassium Sulfate435 mgH2O 20 mL
[0138]Component E: Concentrated (50 mM) Tl2SO4 Solution: 1×20 mL
Per vial50 mM Thallium Sulfate505 mgH2O 20 mL
[0139]Component F: 100×: 1×1 mL
Per vialPLURONIC F12720-100mgPLURONIC P855-20mgH2O1mL
[0140]Cells comprising an ion channel are cultured to about 75% confluence and in log-phase growth. The cells are t...
example 2
Assay of Cells Transfected with hERG Ion Channels
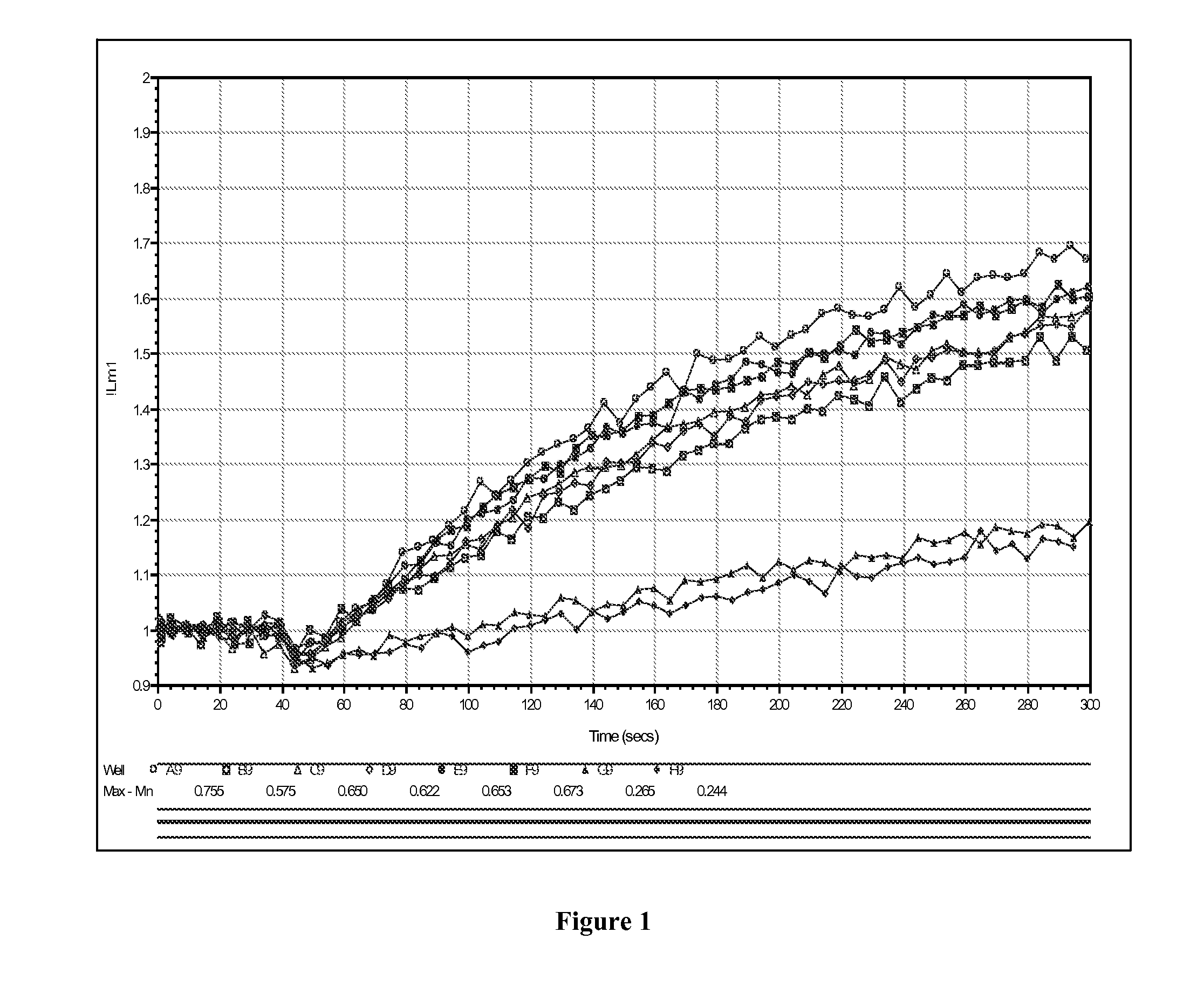
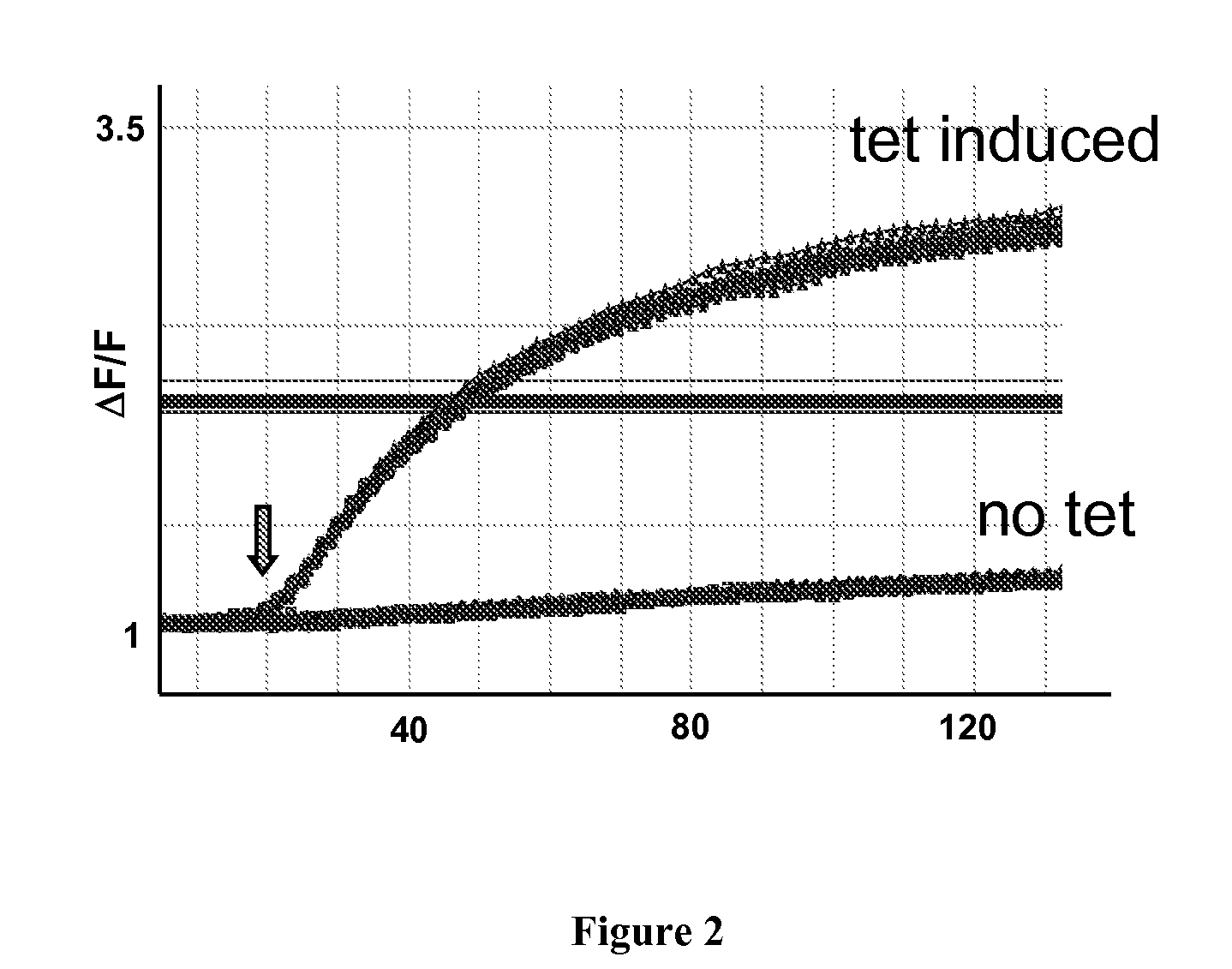
[0147]Cells transfected with the hERG ion channels were assayed using the methods of the present invention. Cells were loaded in assay buffer containing 0.5 uM FluoZin-1 AM ester and stimulated with 1× dilution of stimulus buffer (Component C, containing 25 mM K2SO4 and 10 mM Tl2SO4). “Control” were tet-stimulated hERG 293 T Rex cells, and “no tet” traces were cells which were not stimulated to express hERG. The stimulus was delivered to the cells in a volume of 25 uL, added to 100 uL for a 5× dilution. In this particular example, the final concentration of thallium on the cells was about 4 mM. FIG. 1 demonstrates the absorbance curves of cells (plotted as dF / F as a function of time) transfected with the hERG ion channel and assayed using the methods of the present invention. FIG. 2 depicts hERG activity cells transfected with hERG ion channels (tetracycline induced and non-tetracycline induced) measured in a 96 well plate with the FL...
example 3
Thallium Sensitivity Assay
[0148]An assay is provided to evaluate a compound's sensitivity to thallium (I). Compounds with sufficient sensitivity to thallium (I) may be used as thallium indicators for measuring thallium influx and efflux through ion channels. A 10 micromolar solution of a fluorescent compound is prepared in 10 mM HEPES buffer, pH 7.4. The maximum fluorescence emission intensity is measured by excitation at the compound's excitation maximum wavelength. The measurement is performed in a cuvette in spectrofluorimeter or in a microplate well in a fluorescence microplate reader. Increasing amounts of a stock aqueous solution of thallium sulfate are added to the fluorescent compound solution, such that the final concentration of thallium (I) is 0, 5, 10, 50, 100, 500, 1000, and 2000 micromolar. After each addition of thallium sulfate solution, the fluorescence intensity is measured and compared with the intensity obtained in the presence of zero thallium (I). An overall fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com