Recovering Metal Values from a Metalliferrous Material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0095](a) Solubilization of Metalliferrous Material: H2SO4 / SO2 Leach
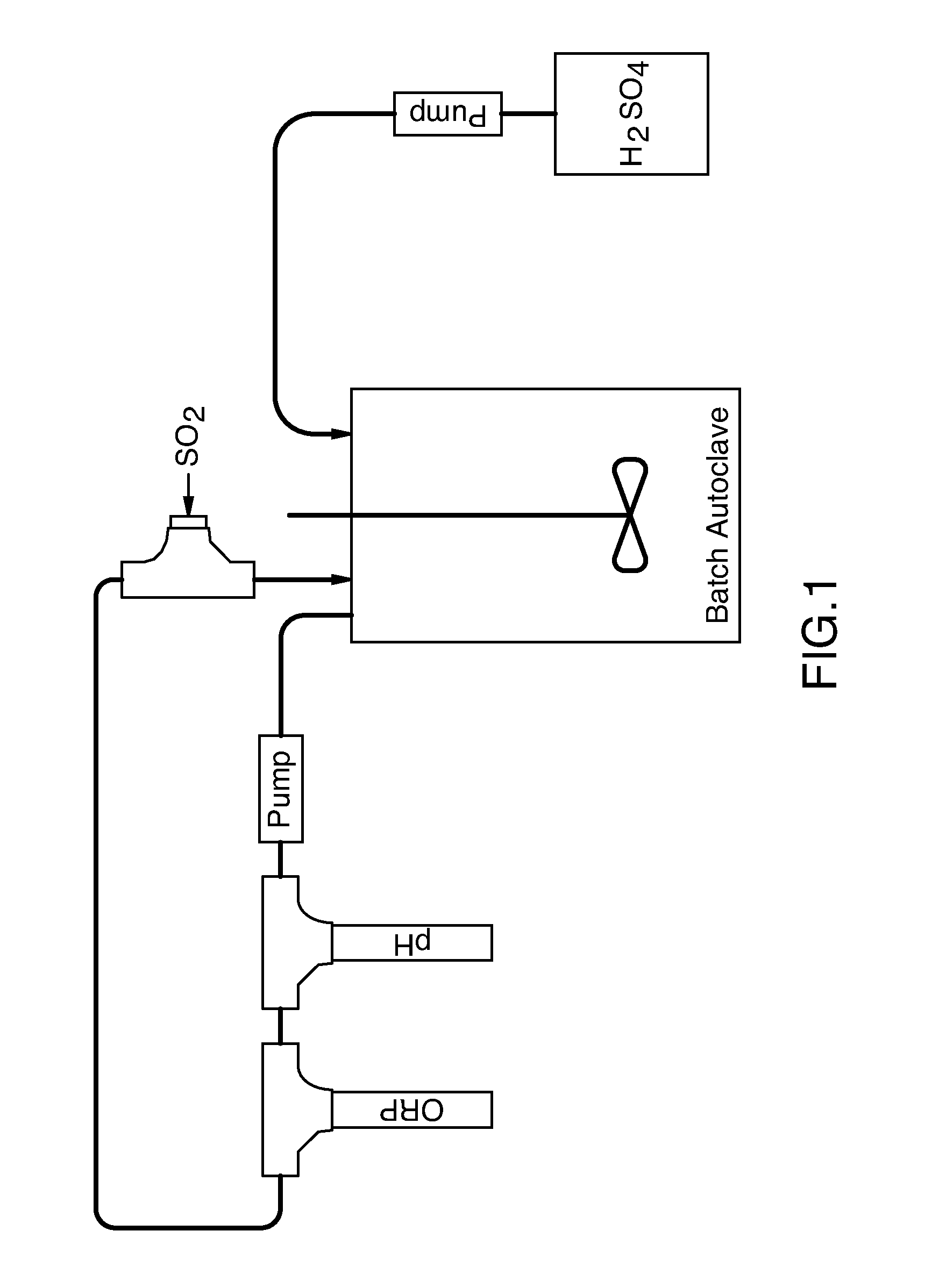
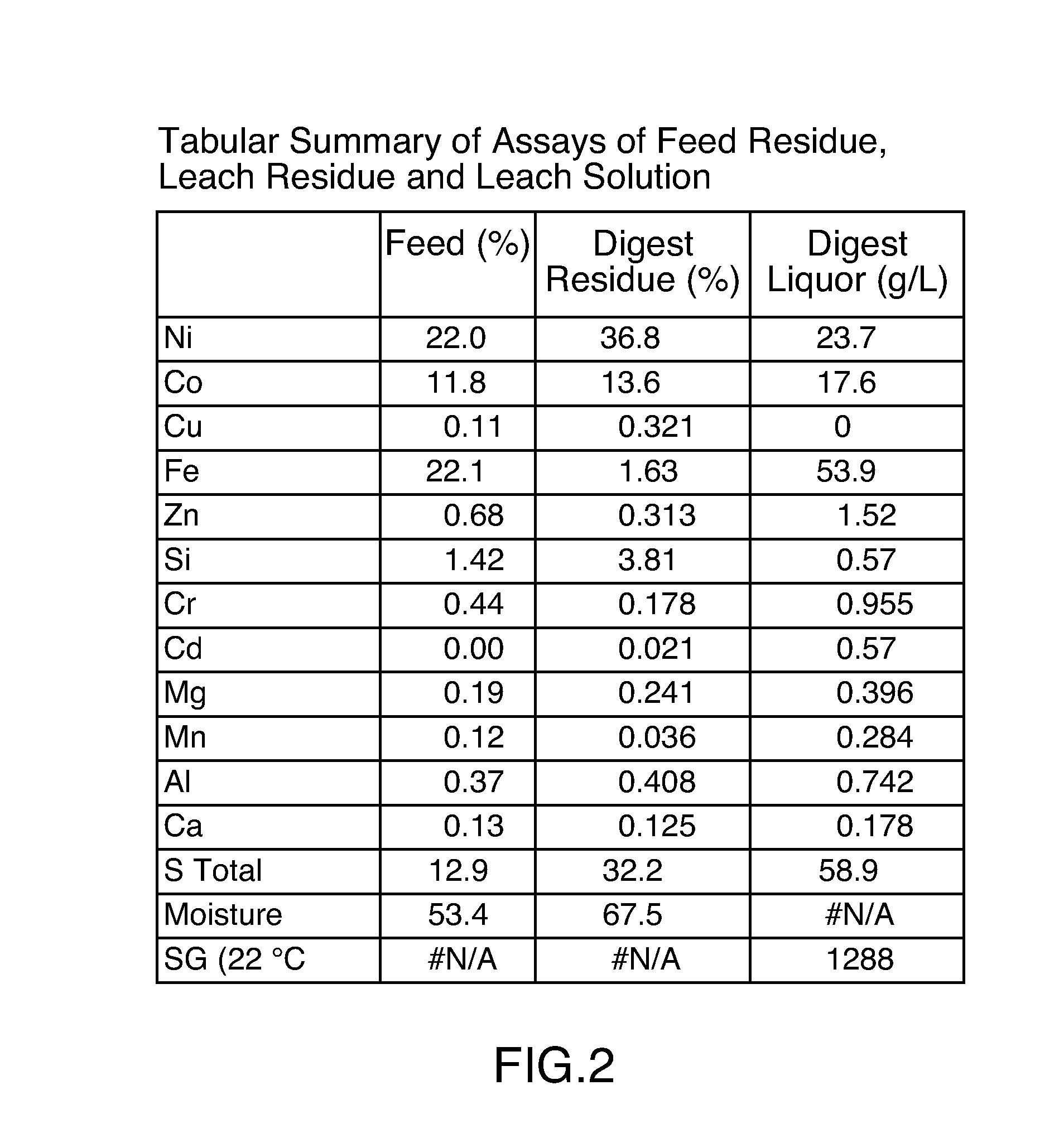
[0096]1600 grams wet filter cake (53.4% moisture), the composition of which is specified in Table 1, prepared from a lamellae thickener underflow were combined with 1600 mls of water in a 3.8 liter titanium lined batch autoclave. The autoclave was equipped with a recirculation loop into which sulphur dioxide gas could be injected. The recirculation loop was also equipped with Oxidation-reduction potential (“ORP”) and pH electrodes. The autoclave was also set up so as to allow injection of concentrated sulphuric acid (See FIG. 1).
[0097]The autoclave was sealed and the circulation loop was started at a flow rate of approximately 350 mls / min. Sulphur dioxide gas was injected into the recirculation loop at a rate of approximately 275 to 300 mls / min. Sulphuric acid was injected into the autoclave so as to maintain a pH of 1.8. The ORP and pressure in the autoclave were monitored. In a typical reaction, the ORP would decr...
example 2
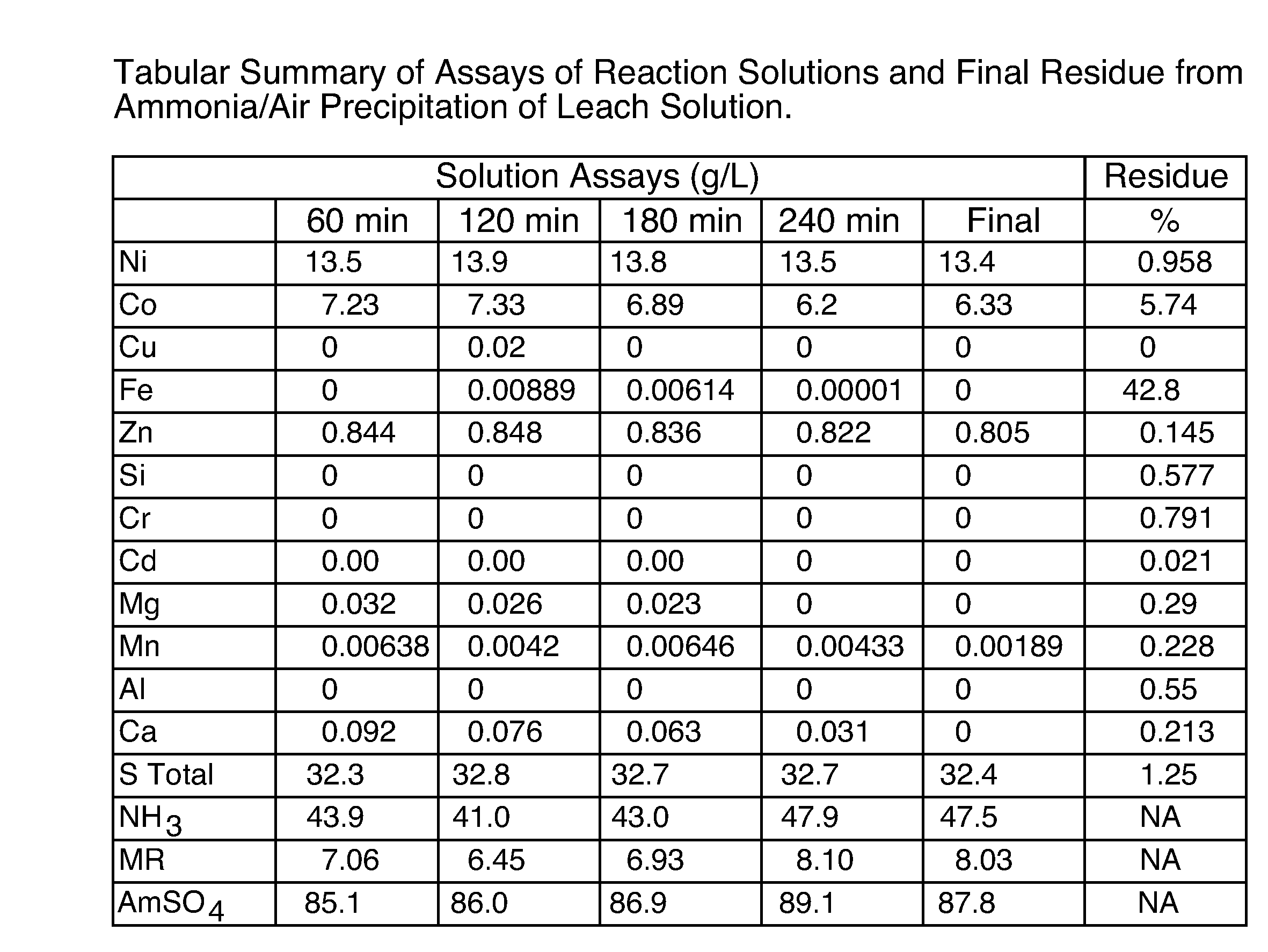
[0105]This example is illustrative of the fact that the subject method can be easily adapted to changes in the composition of the pre-cursor metalliferrous material source. Changes in the composition of the metalliferrous material source and, therefore, the metalliferrous material, can have an impact on the mass balance of the process. This is because there are two paths by which some of the target elemental metals (eg. nickel and cobalt) can be recovered. If the target elemental metal is sulphidic, then it is not solubilized when the metalliferrous material is leached with the aqueous reductant and, therefore, is recovered by recycling the leach residue to the step which effects leaching of the process feed material (including the metalliferrous material source). If the target elemental metal is oxidic, it is recovered by leaching the metalliferrous material with an aqueous reductant.
[0106]Experiments were conducted using the experimental set-up for Example 1, using a metalliferrou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com