Partial peptide of survivin presented on mhc class ii molecule and use therof
a technology of survivin and peptide, which is applied in the field of antigenic polypeptide, can solve the problem that cancer immunotherapy cannot be said to be promising, and achieve the effect of promoting the production of cytokines, stable and inexpensiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
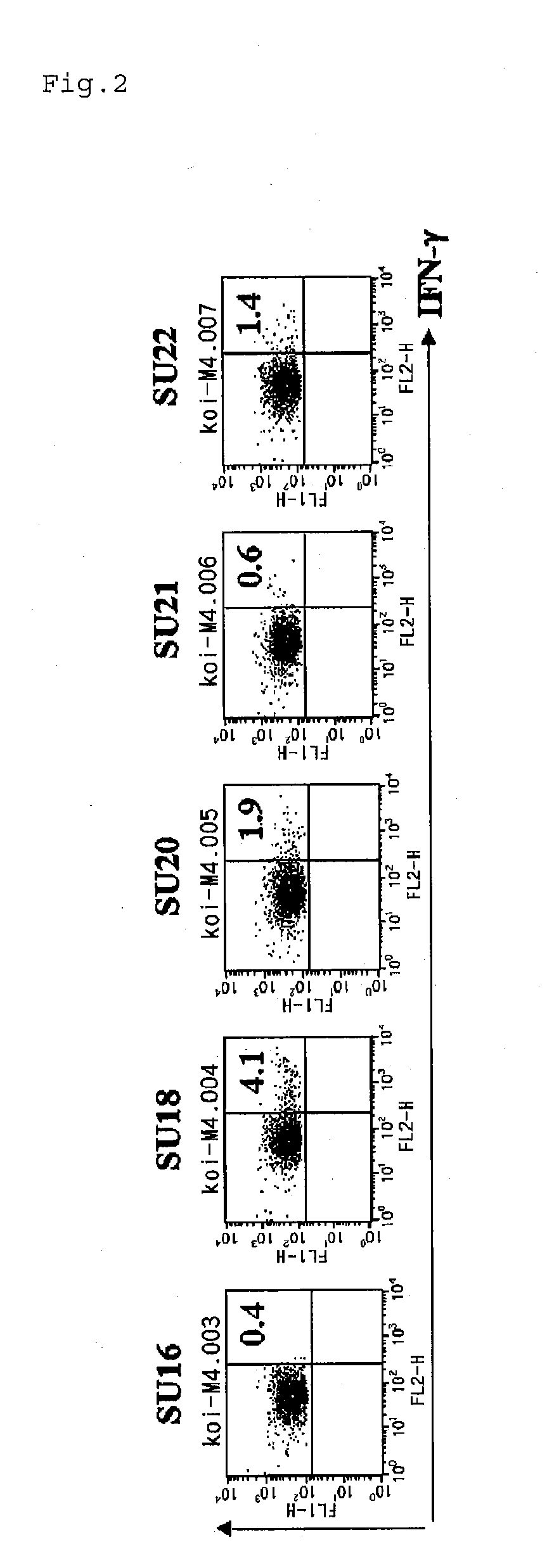
Identification of SU18
1) Synthesis of Peptide
[0074]From the N terminus to C terminus of Survivin, total of 25 types (referred to as SU1 to SU27) of 19 to 20-amino acid residue peptide (Table 1) having an overlapping sequence of 7 to 8 amino acids at the C terminus and / or N terminus were designed, for example, a peptide composed of 1st to 20th amino acid sequence of Survivin-2B (SEQ ID NO:56), a peptide composed of 8th to 27th amino acid sequence thereof, and a peptide composed of 14th to 34th amino acid sequence thereof, each of which was chemically synthesized.
TABLE 1MIX1SU1MGAPTLPPAWQPFLKDHRISSequence No. 1SU2PAWQPFLKDHRISTFKNWPFSequence No. 2SU3LKDHRISTFKNWPFLEGCASequence No. 3SU4FKNWPFLEGCACTPERMAEASequence No. 4SU5EGCACTPERMAEAGFIHCPSequence No. 5MIX2SU6PERMAEAGFIHCPTENEPDLSequence No. 6SU7GFIHCPTENEPDLAQCFSequence No. 7SU8PTENEPDLAQCFFCFKELESequence No. 8SU9DLAQCFFCFKELEGWEPDSequence No. 9SU10FFCFKELEGWEPDDDPIGSequence No. 10MIX3SU11ELEGWEPDDDPIGPGTVAYASequence No. 11SU12DDDPI...
example 2
Examination of HLA-DRB1*0101-Restricted SU18 Recognition Site
1) Confirmation of HLA-Restrictivity
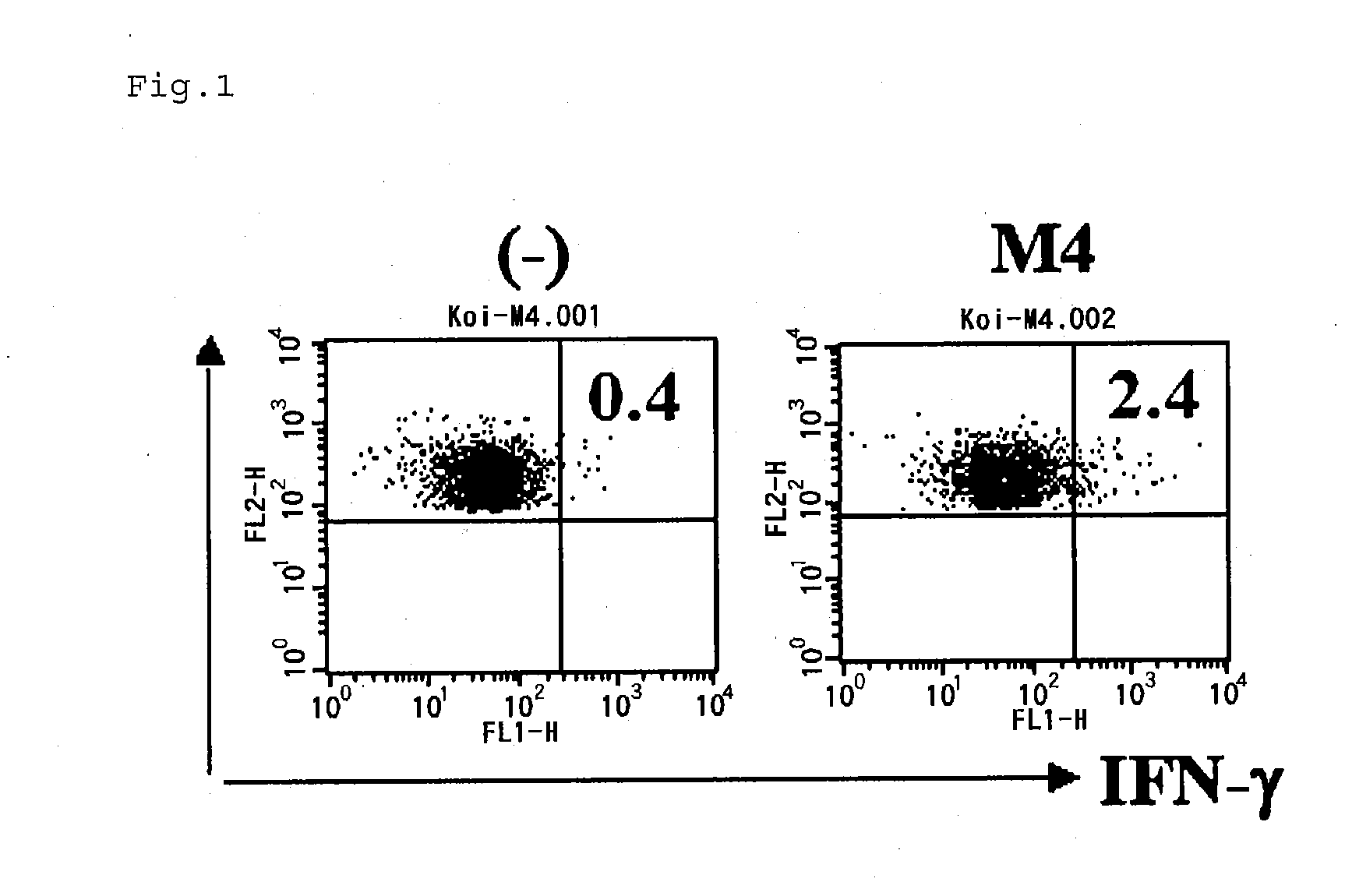
[0084]HLA-restrictivity for SU18 was confirmed by using inhibitory antibodies and the Th cell group obtained in Example 1-4) comprising Sur / Th cells which react specifically with SU18.
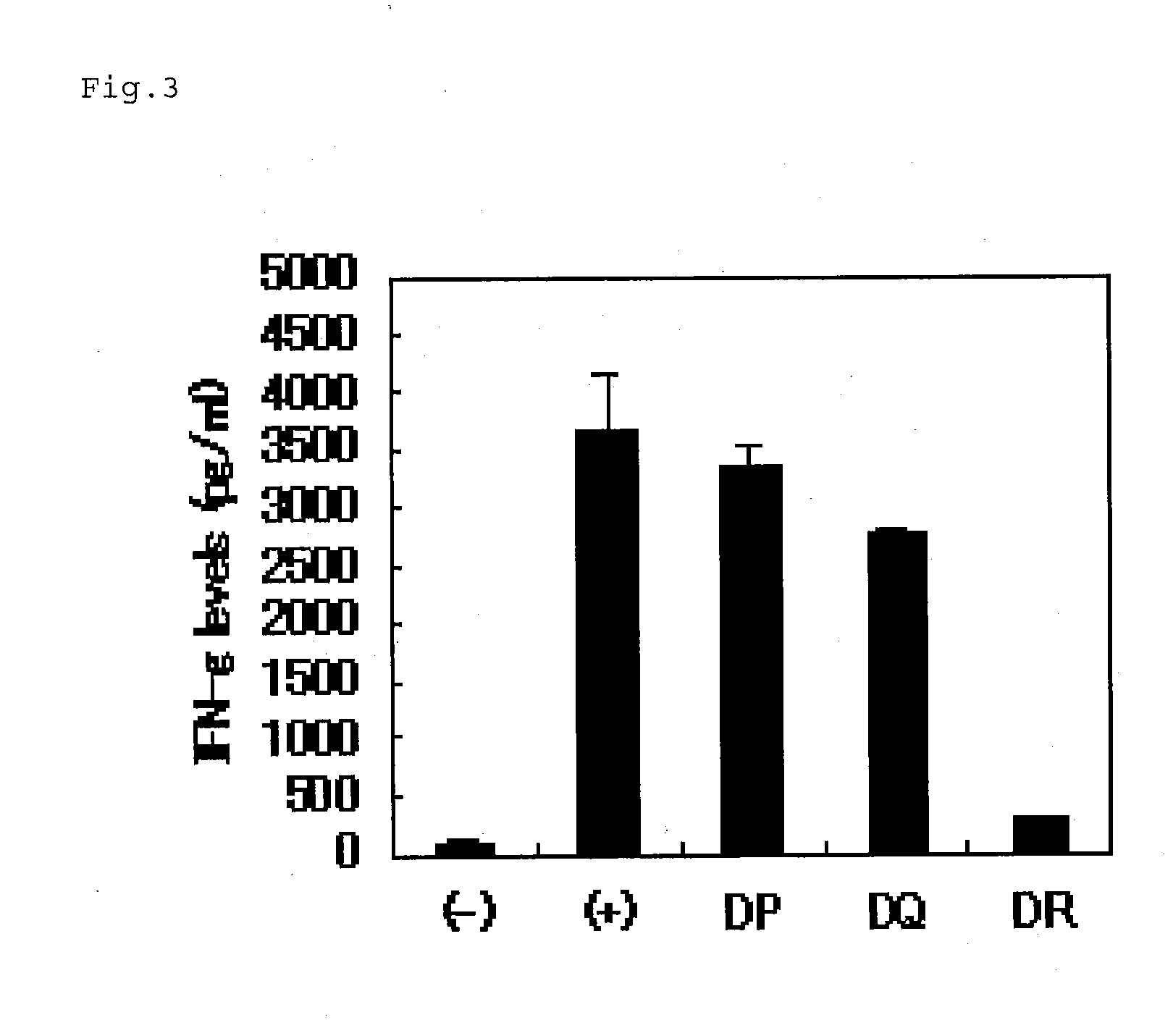
[0085]In a 96-well U-bottom plate (BD Biosciences) with 200 μL of 5% human serum in AIM-V, each of anti-HLA-DP antibodies (Serotech), anti-HLA-DQ antibodies (Serotech) and anti-HLA-DR antibodies (BD Biosciences) was added, to a final concentration of 5 μg / mL, to the PBMCs (1×105 cells / well) and the above-described Th cell group (5'104 cell / well) comprising Sur / Th cells which react specifically with SU18. The resultants were co-cultured in the presence of SU18 peptides in a CO2 incubator at 37° C. for 24 hours. After the culturing, the IFN-γ contained in the culture supernatant was measured by using an ELISA kit (BD Biosciences). The results are shown in FIG. 3.
[0086]As shown in FIG. 3, the inhibition of IFN...
example 3
Examination of HLA-DRB1*1201 / HLA-DRB1*1502-Restricted SU18 Recognition Site
1) Establishment of Th Cell Group Comprising Sur / Th Cells
[0093]By the 28-day co-culture according to the method described in Example 1-2) and 3), a Th cell group comprising Sur / Th cells was prepared from peripheral blood of a healthy individual whose HLA genotype is HLA-DRB1*1201 / HLA-DRB1*1502. Further, to establish a single cell group, a 96-well U-bottom plate was provided with the culture containing 200 μL of the complex medium of human serum and fetal calf serum (2.5% human serum, 2.5% fetal calf serum in AIM-V), PBMCs-Ad (5×104 cells / well), recombinant IL-2 (final concentration of 20U / mL), recombinant IL-7 (final concentration of 10 ng / mL), and phytohemagglutinin (PHA, SEIKAGAKU Co., final concentration of 5 μg / mL). The Th cell group (1 cell / well) was added thereto and the resultant was co-cultured in a CO2 incubator at 37° C.
[0094]After 14 days from the start of the co-culture, the wells which showed bla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com