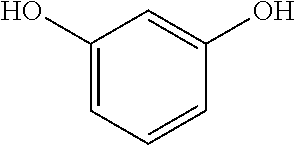
COMPOSITIONS COMPRISING AN NFkB-INHIBITOR AND A TROPOELASTIN PROMOTER
a technology of nfkb-inhibitor and promoter, which is applied in the direction of biocide, plant/algae/fungi/lichens ingredients, drug compositions, etc., can solve the problems that the skin can be aging and affect the elasticity and strength of the skin,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
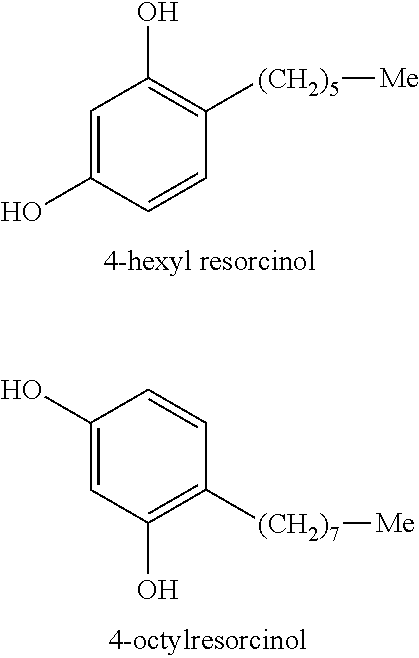
[0086]The NFκB-INHIBITION TEST described above was performed on test samples of Bay 11-7082 (Sigma-Aldrich, St. Louis, Mo.), Tetrahydrocurcuminoids CG (Sabinsa Corporation, Piscataway, N.J.), as well as various concentrations of 4-hexylresorcinol. The results are shown in Table 1, in which NF-kB Gene Reporter Activation (Luminescence, L) is reported for the test samples and a control sample. Percent NF-kB Inhibition is also reported.
TABLE 1NF-kB Gene ReporterPercentActivationNF-kB(Luminescence, L)InhibitionUntreated 1.2 ± 0.3—TNFα (100 ng / ml) Stimulated,108.2 ± 8.5 —“Lcontrol”TNFα + 4-Hexylresorcinol 9.3 ± 0.991.4%(50 ug / ml)TNFα + 4-Hexylresorcinol29.3 ± 9.272.9%(10 ug / ml)TNFα + 4-Hexylresorcinol55.1 ± 1.750.9%(5 ug / ml)TNFα + 4-Hexylresorcinol106.1 ± 1.9 1.9%(1 ug / ml)TNFα + Tetrahydrocurcuminoids CG37.8 ± 2.665.1%(10 ug / ml)Bay 11-7082 (25 uM)11.3 ± 5.689.5%
[0087]Bay 11-7082 and Tetrahydrocurcuminoids CG showed strong NF-kB inhibition. Unexpectedly, 4-hexylresorcinol also resulted i...
example 2
[0088]The NFκB-INHIBITION TEST described above was performed on a series of substituted resorcinols each having a concentration of 10 ug / ml. The results are shown in Table 2.
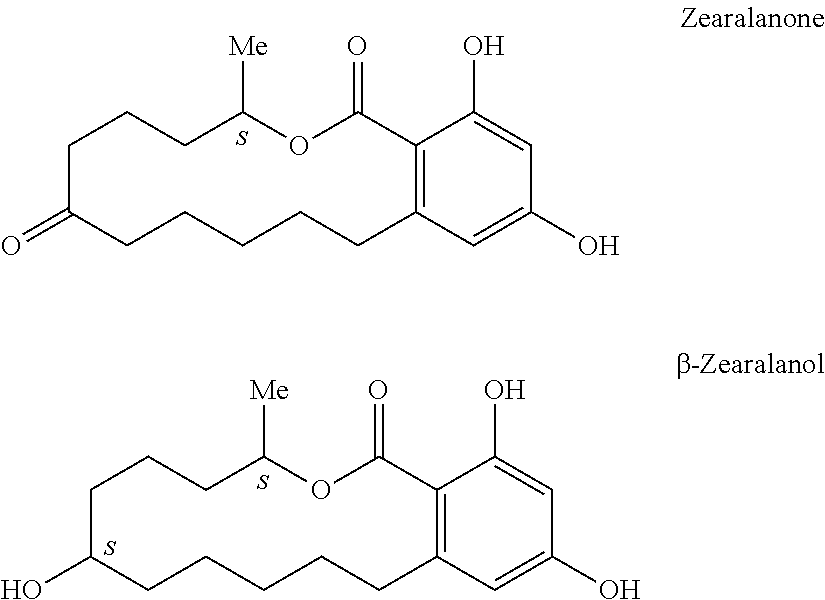
TABLE 2Percent NF-kBStructureInhibition4-Octylresorcinol99.5%4-Hexylresorcinol92.4%β-Zearalenol CAS#71030-11-087.1%β-Zearalanol CAS#42422-68-476.56%2,4-Dinitrosorcinol51.78%4-Chlororesorcinol51.63%2,6-Dichlororesorcinol51.54%Zearalanone50.95%Phenethylresorcinol31.8%4-Dodecylresorcinol20.87%4-Caproylresorcinol10.25%C-Undecylcalix[4]- resorcinarene 4.87%3-Methoxyphenol 0%2′4′- Dihydroxypropiophenone−0.7%2,4- DIHYDROXYCINNAMIC Acid−1.7%1,3-Dimethoxybenzene−1.7%
[0089]It can be seen from the data in Table 2 that superior NFκB inhibition is provided by substituted resorcinols containing only substituents free of phenyl functionalities, substituted resorcinols containing only substituents free of ketone functionalities, and substituted resorcinols comprising a substituent having 5 to 11 carbon atoms.
example 3
[0090]The TROPOELASTIN PROMOTER ASSAY was performed on the following compounds: Tanacetum parthenium (parthenolide-free feverfew extract from Integrated Botanical Technologies of Ossining, N.Y.), Rubus fruticosus (SymMatrix, from Symrise), cells treated with various preparations of Phyllanthus niruri (Raintree Nutrition, Inc., Carson City, Nev.) subsequently extracted with water and fractionated to include only species with molecular weight less than 100,000 daltons, and 4-hexylresorcinol (Synovea HR, Sytheon Ltd).
[0091]The compounds were diluted in cell culture media (DMEM Media of Invitrogen, San Diego Calif.) to the concentration of “active” indicated in Table 3 below. The compounds were added to the transfected H9C2 cells and were incubated for 24 hours. Test samples were compared to a DMSO vehicle.
[0092]The results are shown in Table 3.
TABLE 3RespectivePercentRatio ofConcentrationsTropoelastinChangeNFκB-Inhibitor:of ActivesPromoterOverTropoelastinCompound / Extract(on active basi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration by weight ratio | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com