Cell-based systems for producing influenza vaccines
a cell-based system and vaccine technology, applied in viruses/bacteriophages, transferases, antibody medical ingredients, etc., can solve the problems of 2004 worldwide influenza vaccine shortage, difficult automation of process, significant risk of contamination, etc., and achieve the effect of preventing or minimizing the infection of influenza virus in a human cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ST6 CHO Cells with Increased Levels of 2,6-Linked Sialic Acids and Which Produce Increased Yields of Influenza Virus
[0084]CHO cells were incubated with digoxigenin labeled S. nigra agglutinin (SNA), specific for 2,6-linked sialic acids followed by the anti-digoxigenin-fluorescein conjugated antibody, and then analyzed by flow cytometry. The mean fluorescence intensity of WT CHO indicates that WT CHO do not contain 2,6-linked sialic acids on the cell surface. ST6 CHO cells expressing the human ST6Gal 1 gene however contain increased levels of 2,6-linked sialic acids as shown by the curve shift to the right (increased mean fluorescence intensity). See FIG. 4.
[0085]Next, TC-24 cells were seeded with 2×105 CHO or ST6CHO cells. The confluent CHO or ST6CHO monolayers were infected with A / PR / 8 / 34 influenza virus at an MOI=1 the following day. Aliquots were taken out at 48 hours post infection and stored at −80° C. Viral titers were determined on MDCK cells by plaque assay. Briefly, TC-6 ...
example 2
Generate Stable CHO and Vero Cells Expressing Increased Numbers of Human Influenza Virus Specific Receptors
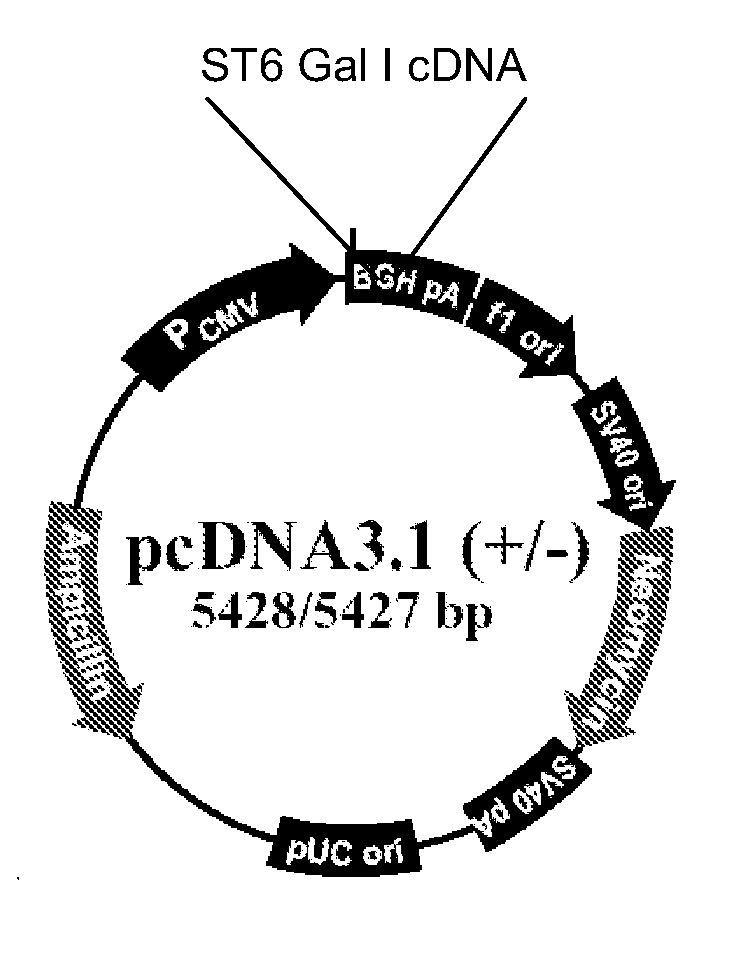
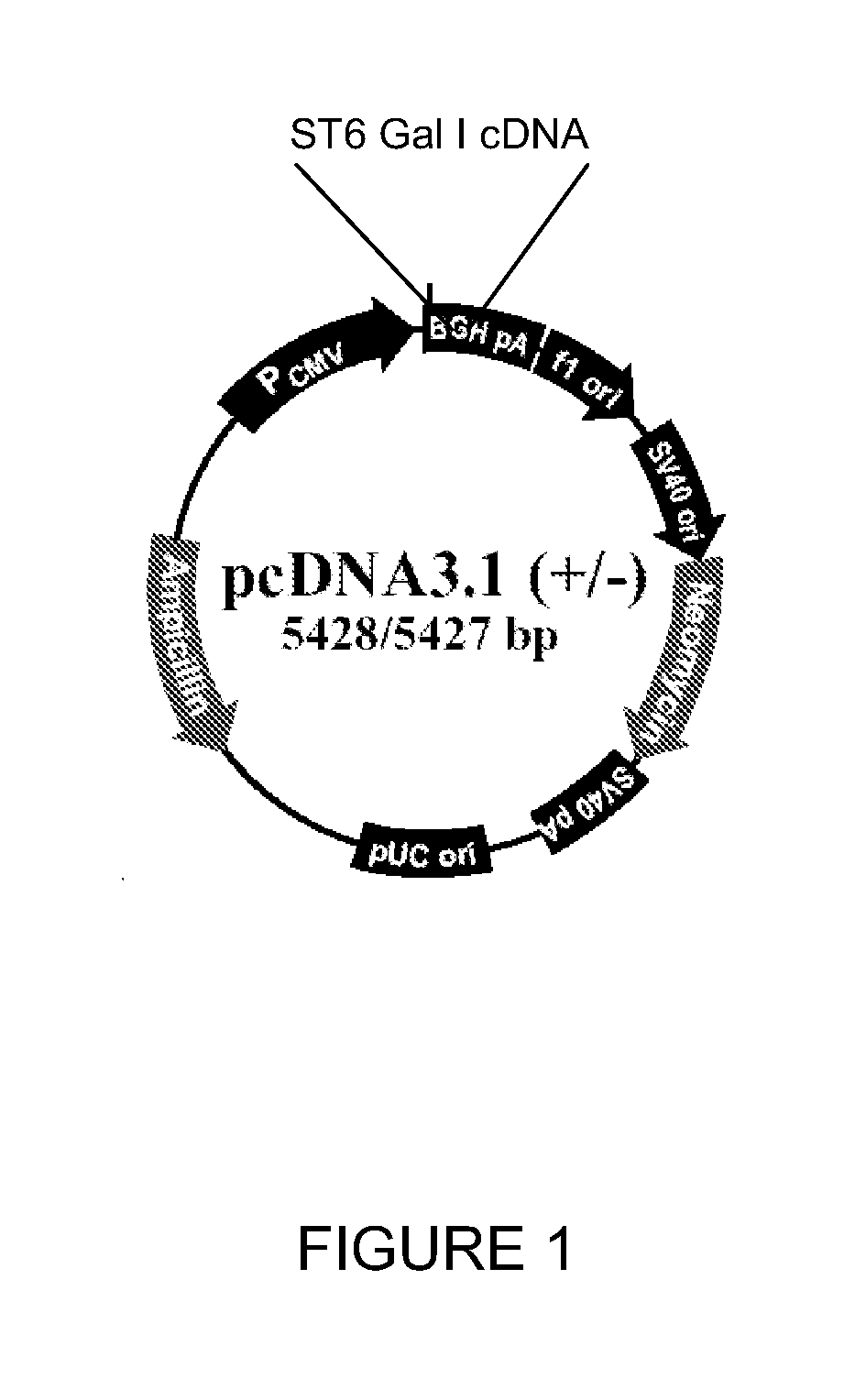
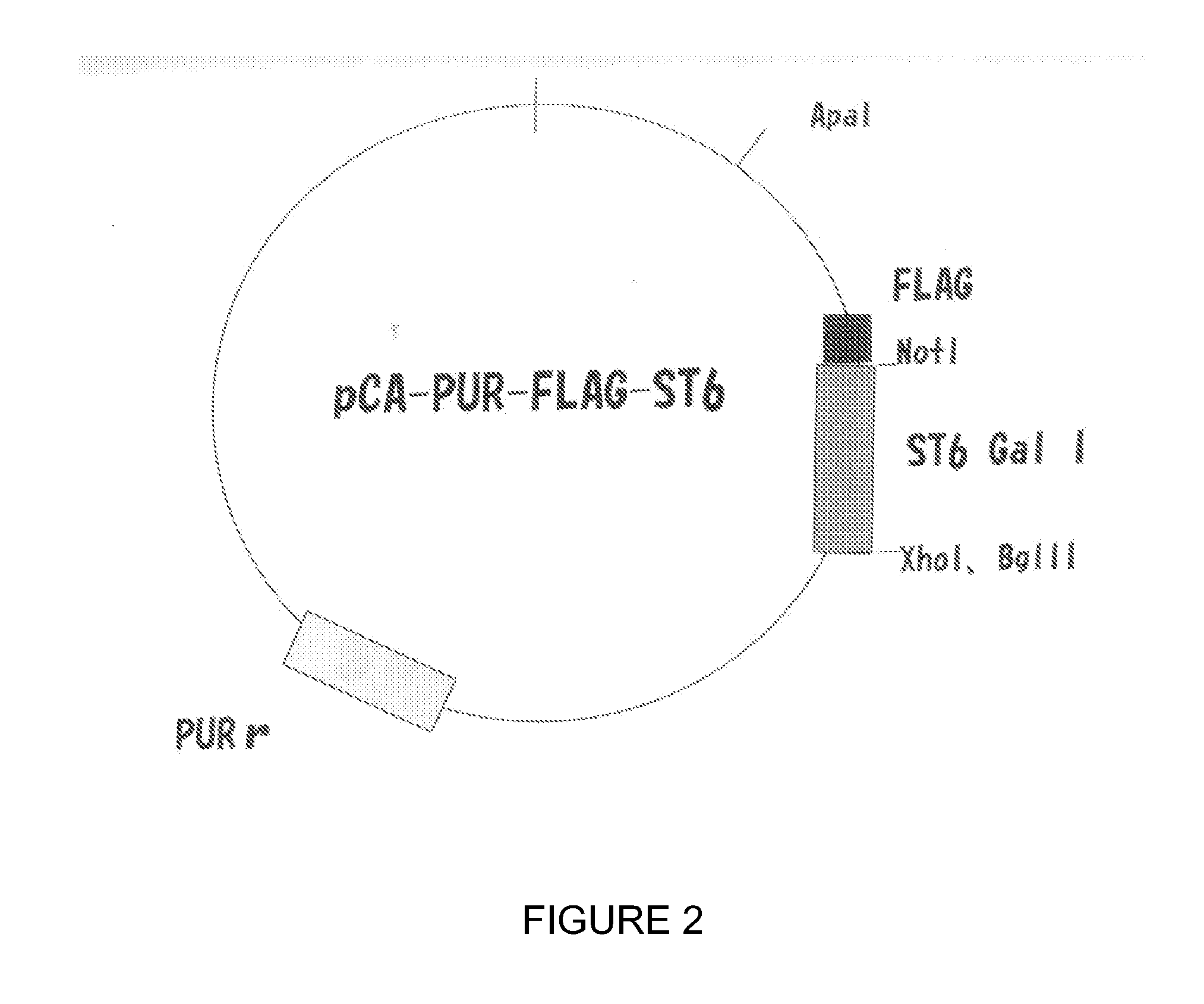
[0088]This example concerns the stable transfection of the ST6Gal I gene, which encodes for 2,6-sialyltransferase I, into the CHO cell genome. This modification is expected to facilitate the production of vaccine virus in CHO cells.
[0089]Cell lines: CHO-S (Cat #11619012, Invitrogen, San Diego, Calif.); Vero (ATCC CCL-81, Manassas, Va.); MDCK (ATCC CCL-34); Plasmids: ST6Gal I (ATCC, Manassas, Va.), pcDNA3.1 (Invitrogen, San Diego, Calif.); Viruses: A / Puerto Rico / 8 / 34 (H1N1), A / Nanchang / 933 / 95 (H3N2), A / Texas / 36 / 91 (H1N1), B / Florida / 4 / 06) (BEI, Manassas, Va.).
[0090]A. Construct Plasmids Expressing the ST6Gal I Gene
[0091]Human (α2,6) ST6Gal I cDNA in the pSPORT vector (Invitrogen) is available from the ATCC (cat #10436251). The ST6Gal I gene was amplified by polymerase chain reaction (PCR) techniques with primers 5′-AAGCTTGCCGCCACCATGATTCACACCAAC-3′ (SEQ ID NO. 2) and 5′-CGGCGCC...
example 3
Quantitate Seasonal and Pandemic Influenza Virus Yields Following Infection of CHO 2,6
[0101]This example will test whether higher expression levels of α2,6-linked sialic acid on the cell surface of the modified cells (CHO-2,6 and Vero-2,6 cells) generated in the preceding Example increases the cell's susceptibility to human influenza virus infection. The α2,6-linked sialic acid is the cell receptor used by the HA glycoprotein on the influenza virus that permits viral attachment to the host cell and leads to infection. The parental and modified CHO and Vero cell lines will be infected with seasonal and pandemic human influenza virus isolates and evaluated for their ability to yield high titers of virus. Populations of all 4 cell lines will be infected with representative influenza viruses (H1N1, H3N2, type B, and H5N1) and growth curves will be generated for each strain. The H1N1, H3N2, and type B influenza viruses represent the different subtypes included in the seasonal influenza ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com