Herbicide combination comprising dimethoxytriazinyl-substituted difluoromethanesulfonylanilides
a technology of difluoromethanesulfonyl and dimethoxytriazinyl, which is applied in the field of crop protection compositions, can solve the problems of chemical, physical or biological incompatibility, lack of joint stability, and inability to safely discount a priori negative or positiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
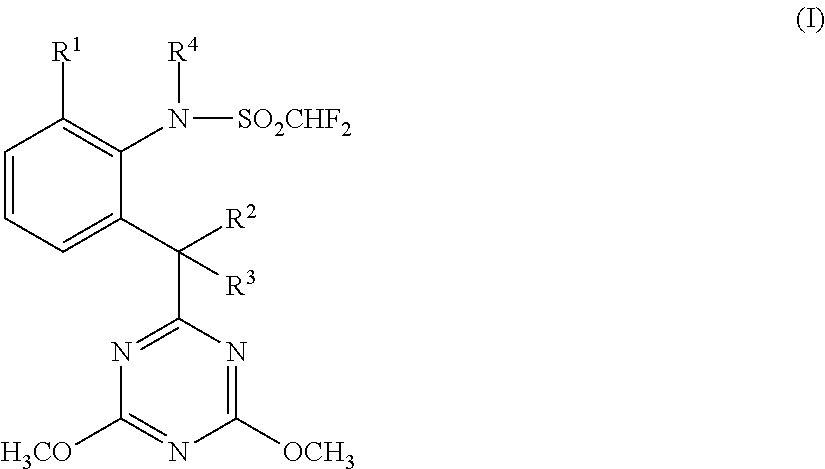
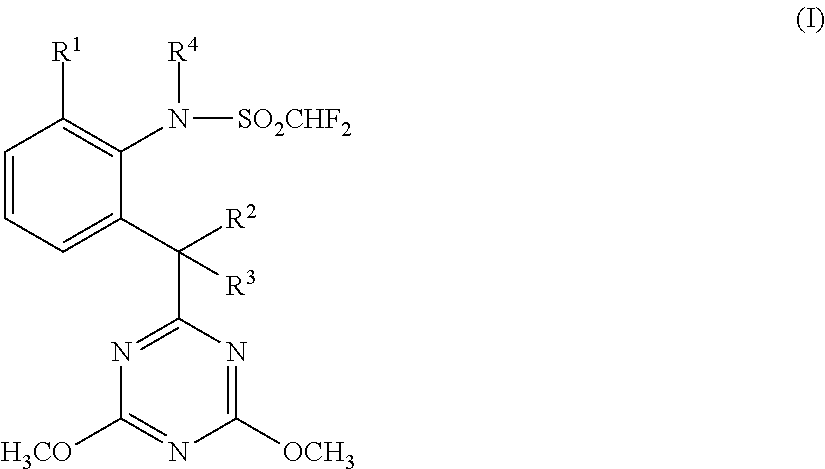
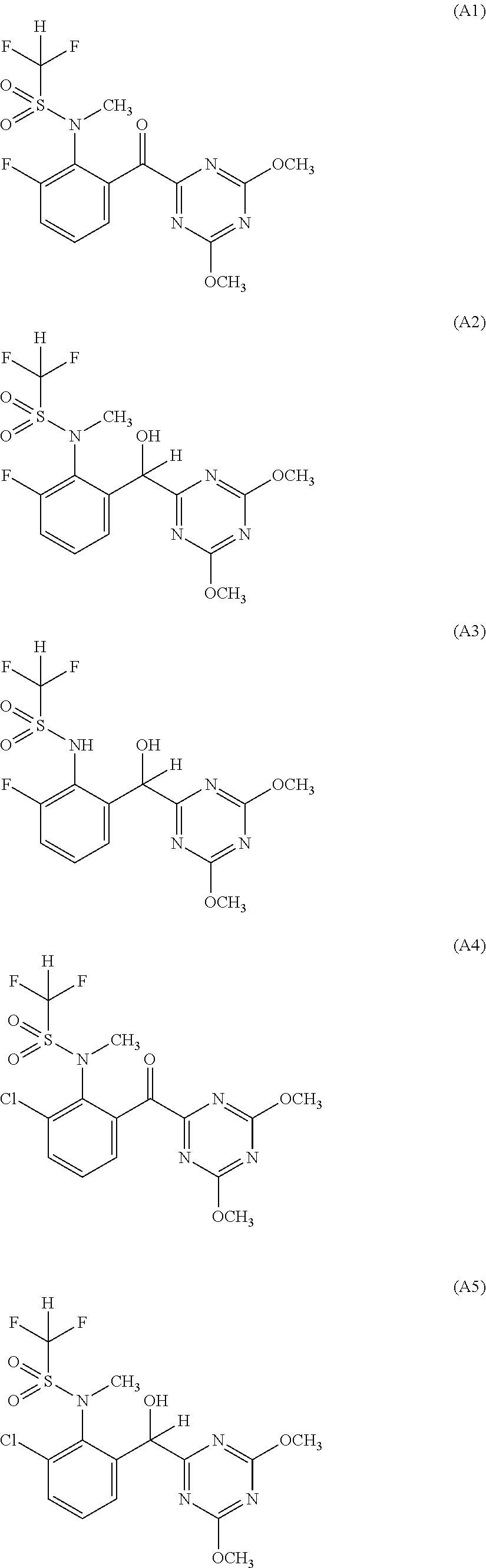
[0015]Accordingly, the present invention provides a herbicide combination comprising components (A) and (B) where[0016](A) denotes one or more compounds or salts thereof from the group described by the general formula (I):
[0017]in which[0018]R1 is halogen, preferably fluorine or chlorine,[0019]R2 is hydrogen and R3 is hydroxyl or[0020]R2 and R3 together with the carbon atom to which they are attached are a carbonyl group C═O and[0021]R4 is hydrogen or methyl;
and[0022](B) denotes one or more herbicides from the group of the pyrimidines consisting of:[0023]the subgroup of the pyrimidinylcarbinols (subgroup 1) consisting of:[0024](B1-1) ancymidol (PM #31), for example α-cyclopropyl-α-(4-methoxyphenyl)-5-pyrimidinemethanol (application rate: 1-5000 g of AS / ha, preferably 3-4000 g of AS / ha; weight ratio A:B=1:5000-500:1, preferably 1:800-70:1);[0025](B1-2) flurprimidol (PM #403), for example α-(1-methylethyl)-α-[4-(trifluoromethoxy)phenyl]-5-pyrimidinemethanol, also including its racemat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com