Differentiation-inducing culture medium additive and use thereof
a technology additive, which is applied in the field of differentiation-inducing culture medium additive, can solve the problems of high cost of serum, difficult to achieve the effect of low cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study on Differentiation of Mesenchymal Stem Cells into Osteoblast Cells (1)
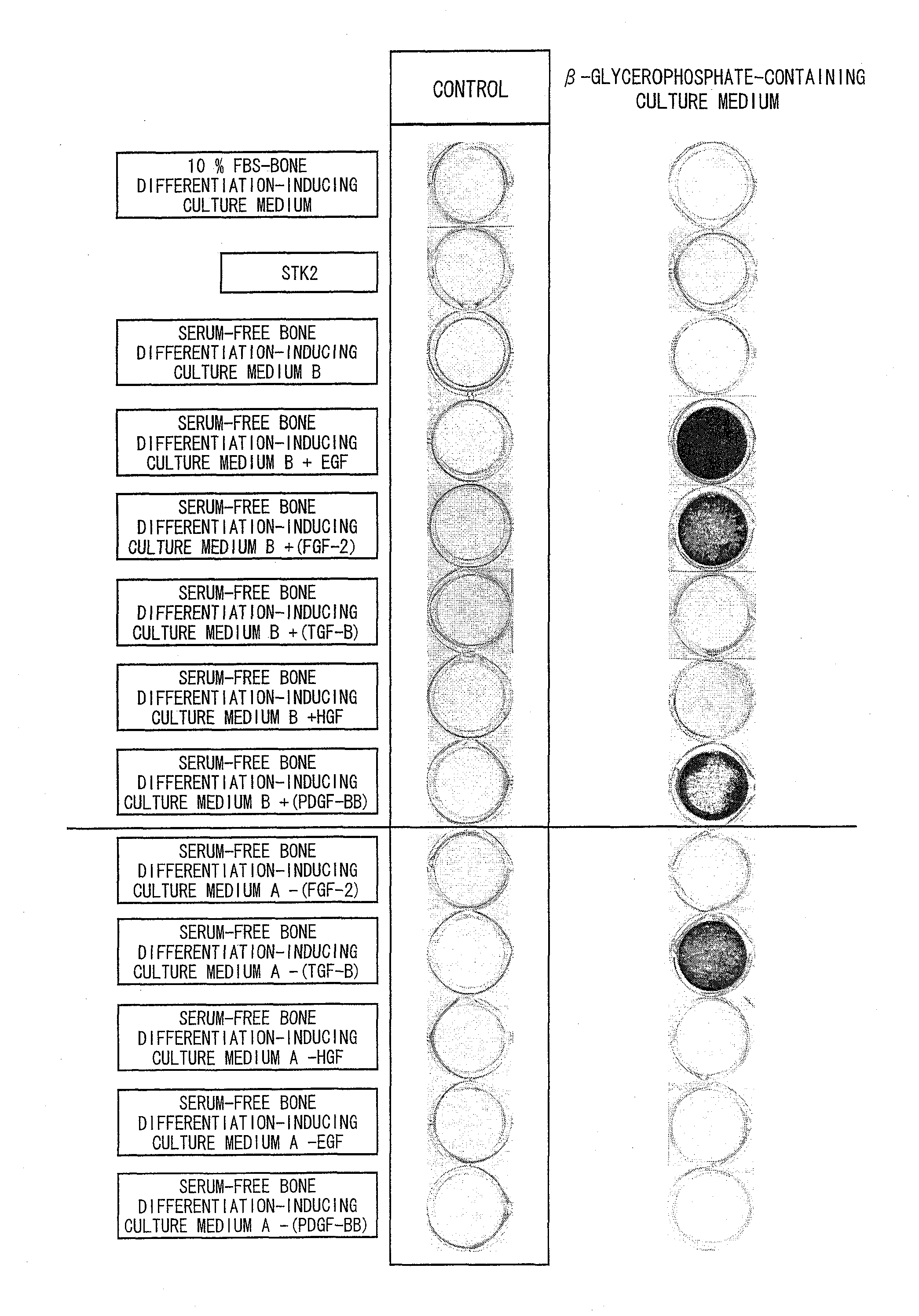
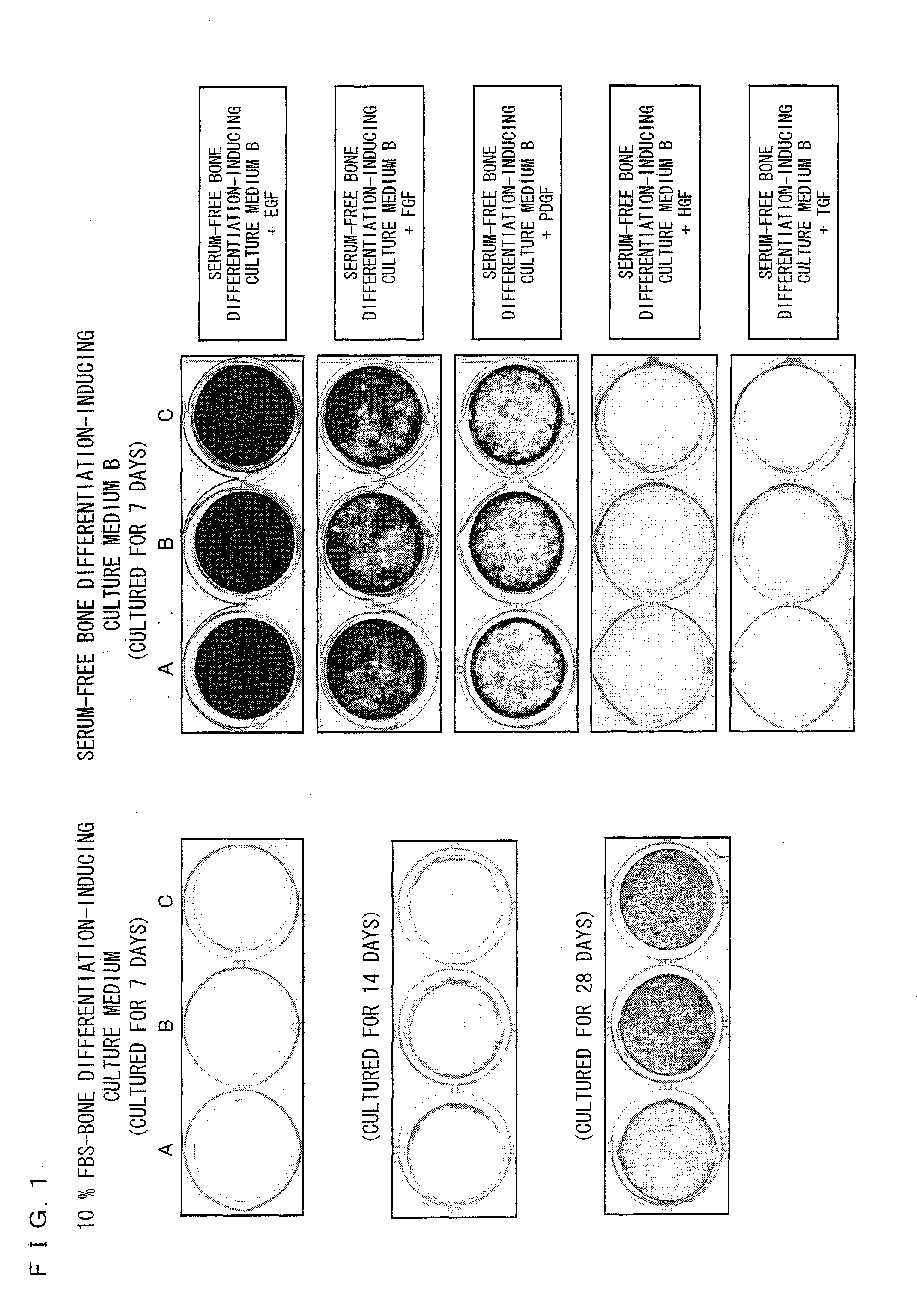
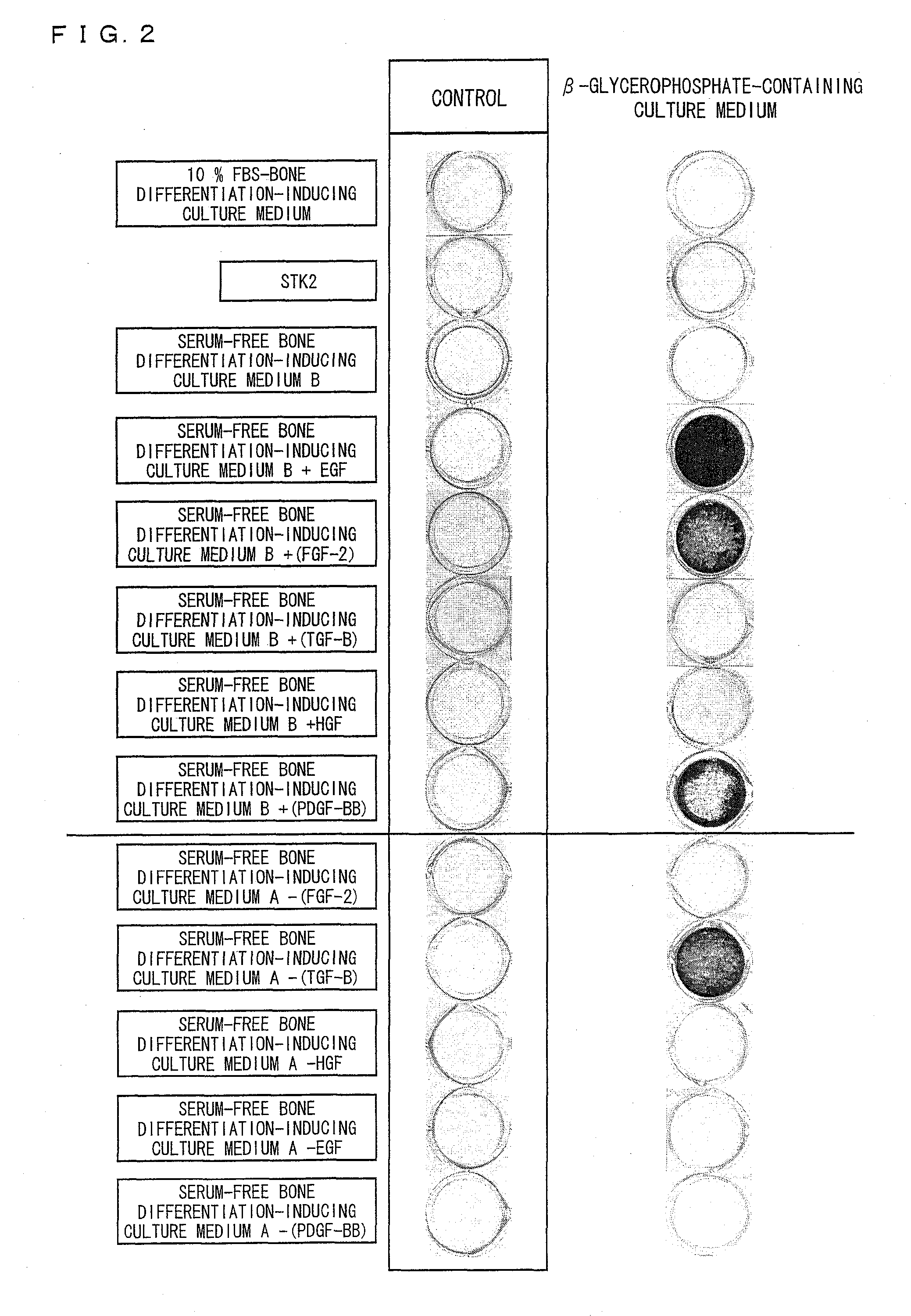
[0130]Three human ilium-marrow-derived mesenchymal stem cell lines (hereinafter referred to as a cell line A, a cell line B, and a cell line C) (purchased from Bio-Whittaker Inc. (Walkersville, Md.)) were used as mesenchymal stem cells. Note that the cell line A is a fifth subculture of human ilium-marrow-derived mesenchymal stem cells and each of the cell lines B and C is a fourth subculture of human ilium-marrow-derived mesenchymal stem cells. A DMEM culture medium containing 10% FBS was used to culture the mesenchymal stem cells. The mesenchymal stem cells were seeded on a 6-well microplate containing the DMEM culture medium at a density of 5000 cells / cm2. Culture media were replaced every two or three days during the cell culture.
[0131]Note that the DMEM culture medium containing 10% FBS was prepared by adding fetal bovine serum (FBS) in a final concentration of 10% by weight to DMEM (produced by Sigma A...
example 2
Study on Differentiation of Mesenchymal Stem Cells into Osteoblast Cells (2)
[0146]Five human ilium-marrow-derived mesenchymal stem cell lines (hereinafter referred to as a cell line F, a cell line G, a cell line H, a cell line I, and a cell line J) (purchased from Bio-Whittaker Inc. (Walkersville, Md.)) were used as mesenchymal stem cells. Note that each of the cell lines F, H, and I is a fifth subculture of human ilium-marrow-derived mesenchymal stem cells and each of the cell lines G and J is a fourth subculture of human ilium-marrow-derived mesenchymal stem cells. A DMEM culture medium containing 10% FBS was used to proliferate and culture the mesenchymal stem cells. The mesenchymal stem cells were seeded on a 6-well microplate containing the DMEM culture medium containing 10% FBS at a density of 5000 cells / cm2. Culture media were replaced every two or three days during the cell culture.
[0147]Before becoming confluent, the mesenchymal stem cells were collected from the 6-well mic...
example 3
Study on Differentiation of Mesenchymal Stem Cells into Osteoblast Cells (3)
[0161]Three human ilium-marrow-derived mesenchymal stem cell lines (hereinafter referred to as a cell line K, a cell line L, and a cell line M) (purchased from Bio-Whittaker Inc. (Walkersville, Md.)) were used as mesenchymal stem cells. Note that the cell line K is an eighth subculture of human ilium-marrow-derived mesenchymal stem cells, the cell line L is a seventh subculture of human ilium-marrow-derived mesenchymal stem cells and the cell line M is a tenth subculture of human ilium-marrow-derived mesenchymal stem cells.
[0162](1. Induction of Bone Differentiation of Mesenchymal Stem Cells Proliferated and Cultured by Use of Serum-Free STK2 Culture Medium)
[0163]The mesenchymal stem cells were seeded on a 6-well microplate containing a serum-free STK2 culture medium at a density of 5000 cells / cm2. Culture media were replaced every two or three days during the cell culture.
[0164]The mesenchymal stem cells we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com