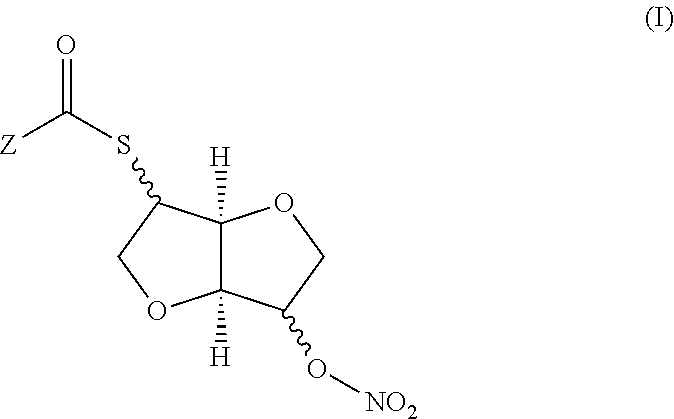
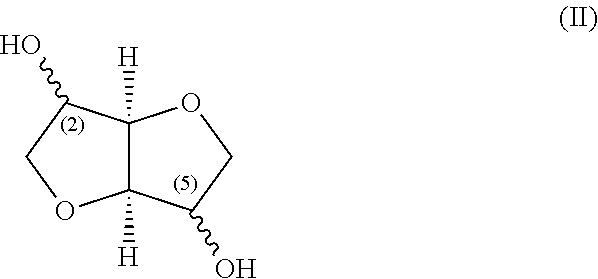
Stereospecific method for the preparation of dioxa-bicyclooctane nitrate compounds
a dioxa-bicyclooctane nitrate and stereospecific technology, applied in the field of new stereospecific method for the preparation of dioxabicyclooctane nitrate compounds, can solve the problems of unsafe nitrating agents, unsatisfactory safety of acetic anhydride, and low yield of the preparation method, so as to achieve the effect of higher overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2-acetylthio-isosorbide 5-mononitrate
Step 1: Preparation of Isosorbide ditriflate
[0044]
[0045]Isosorbide (300 g), dichloromethane (7.5 L, 9.938 Kg) and pyridine (0.586 Kg) are charged to a 20 L flange flask. The resultant solution is cooled (3° C.) with stirring. Trifluoromethanesulfonic anhydride (0.749 L, 1.257 Kg) is added to the solution via dropping funnel.
[0046]The reaction is stirred out to room temperature for 1-2 hours and the reaction is quenched by the cautious addition of water (1.5 Kg).
[0047]The organic layer is removed and the aqueous layer is re-extracted with dichloromethane (0.750 L).
[0048]All organic layers are washed with 5M HCl acid solution and with water until neutral pH.
[0049]The organic layers are charged to 7.5 L of heptane, after that the dichloromethane was removed from the product mixture by distillation using partial vacuum such that vessel temperature does not exceed 50° C.
[0050]The resultant precipitate is cooled to 3° C. stirred for at lea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com