Treating Insulin Secreting Cells
a technology of secreting cells and insulin, applied in the direction of transferases, drug compositions, hormone peptides, etc., can solve the problems of limited therapies, no effective medical treatment for the devastating symptoms, and significant morbidity associated with their removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Demonstration of β-Cell Specific Cytotoxicity Using a Rat Insulin Promoter Thymidine Kinase Construct
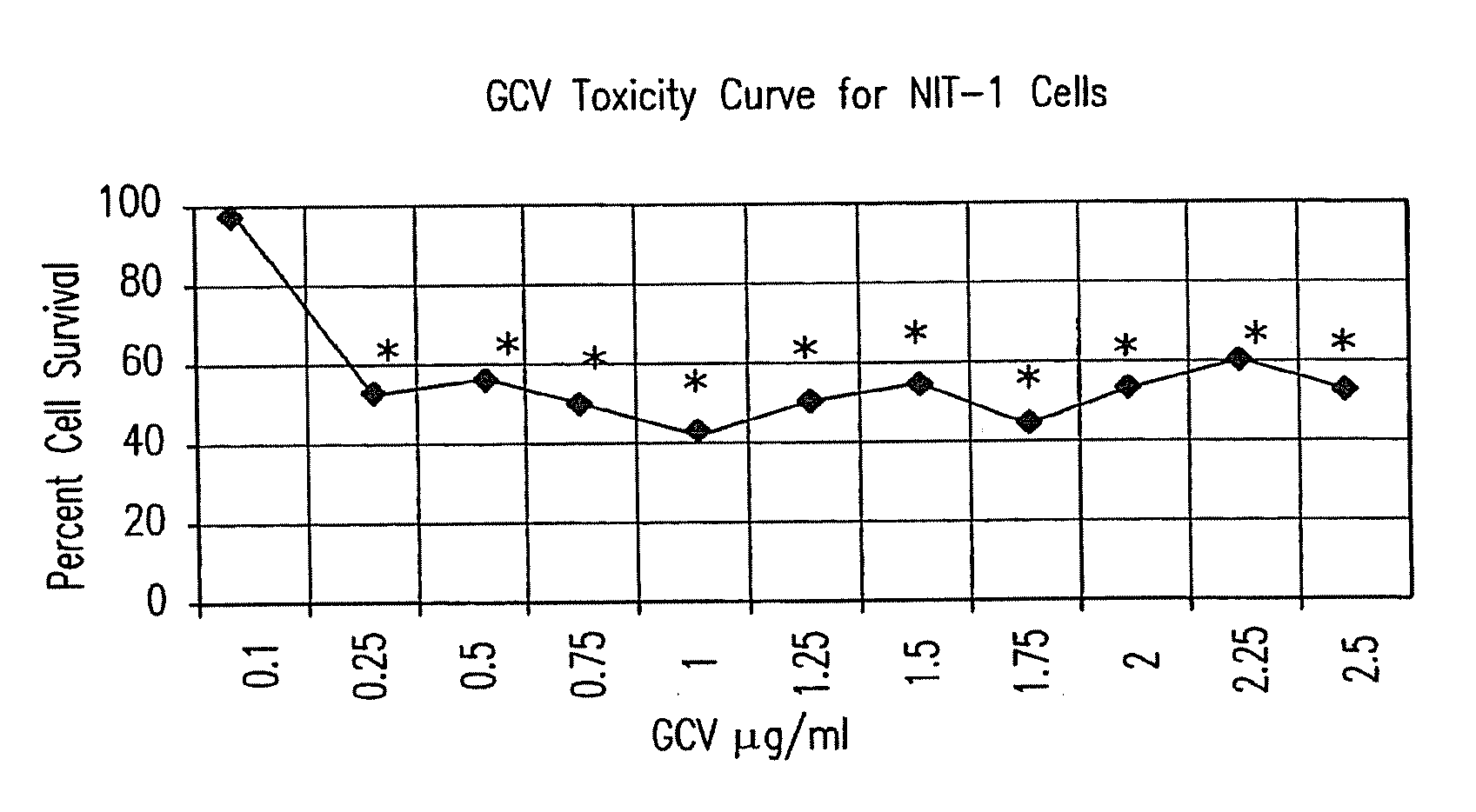
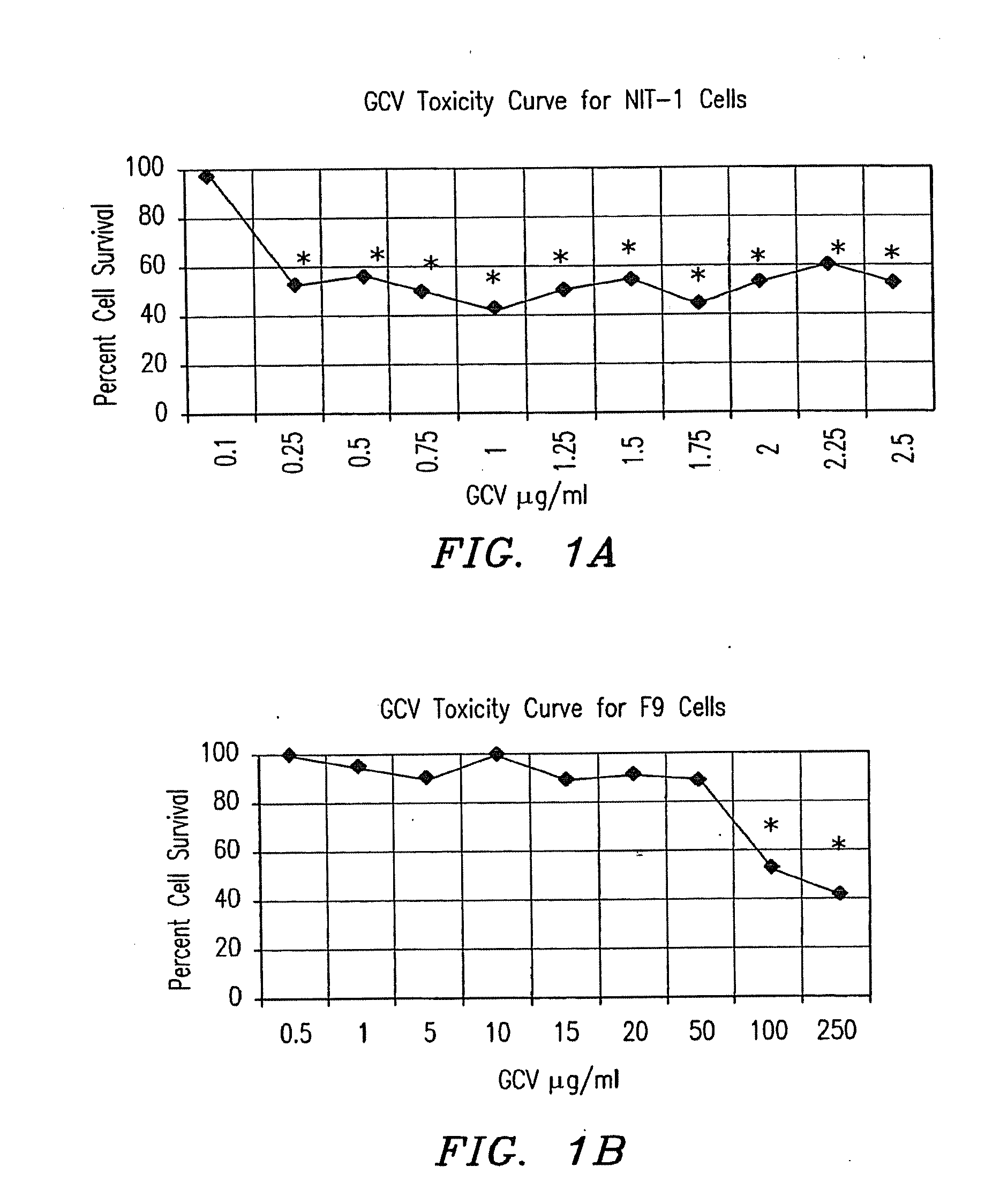
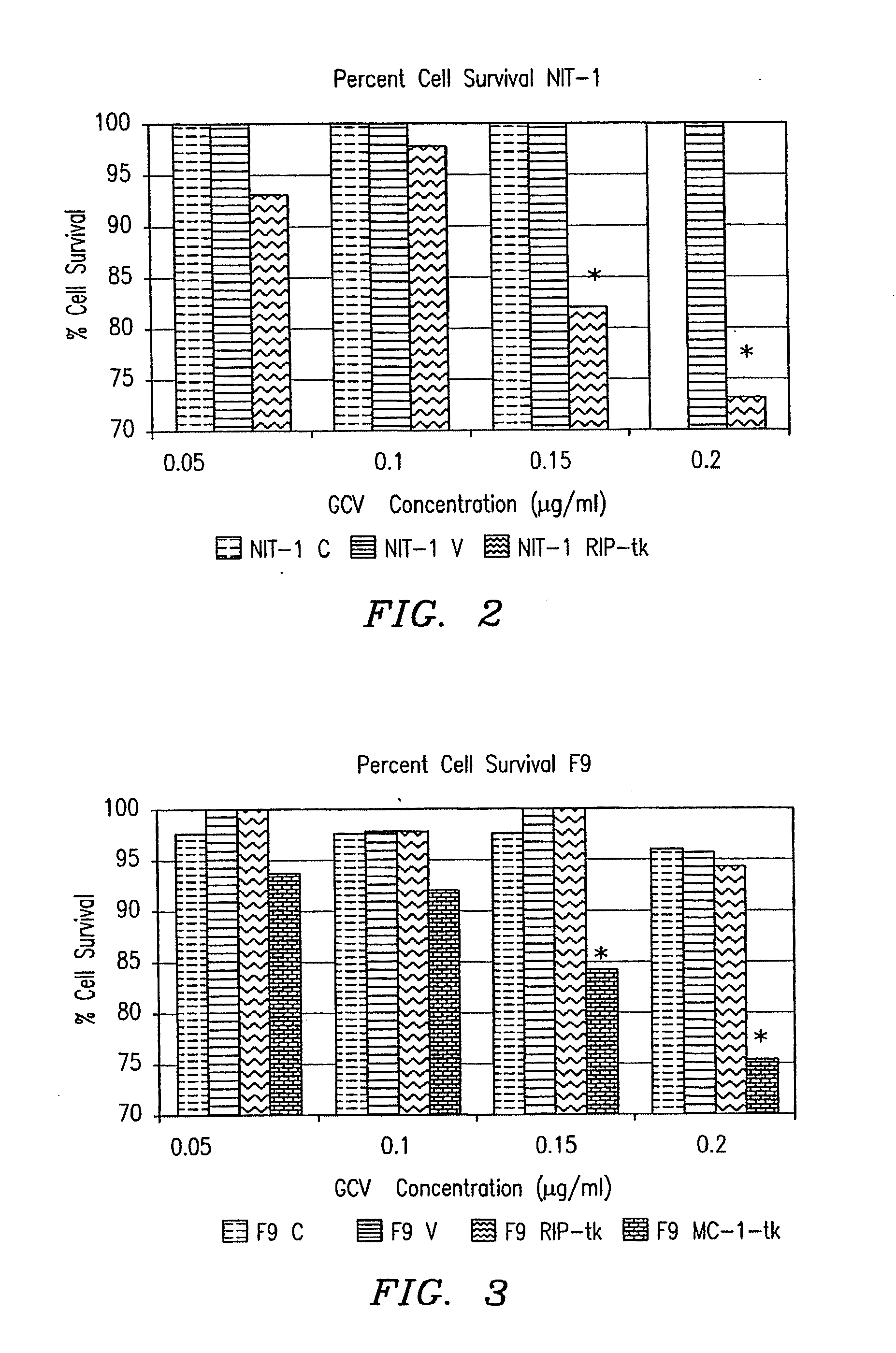
[0105]Materials, methods and summary of results: 0.502 kb of RIP (SEQ ID NO:1) was ligated to the reporter gene LacZ and ligated to tk. These two genes were transfected into several cell lines to ascertain β-cell specific expression and β-cell specific cytotoxicity in vitro. RT-PCR and EMSA were performed on NIT-1 cell RNA and nuclear extract, respectively, to determine the transcription factors present and responsible for RIP activation in NIT-1 cells. A mouse (3-cell adenoma model was created with NIT-1 cells. These mice were treated with the RIP-tk gene and both blood sugars and animal viability were monitored.
[0106]Only the beta (NIT-1) cells stained blue after X-gal staining (p<0.05, n=16) or had detectable levels of beta-galactosidase protein (p<0.05, n=6) in vitro. A significant decrease in cell survival was observed in NIT-1 cells transfected with RIP-tk, in vitro (p<0.05, n=...
example 2
Human Pancreatic Ductal Carcinoma Cells can be Targeted Using a RIP-tk Construct In Vitro
[0135]Materials, methods and summary of results: 0.502 kb of RIP was ligated to the reporter gene LacZ and transfected into several human cell lines: human pancreatic ductal carcinoma cell lines (PANC-1, CAPAN-1, and MIA-1), lung carcinoma (A549), and breast carcinoma (T47D). X-gal staining and the detection of beta-galactosidase using a luminometer analyzed LacZ gene expression. RIP was ligated to tk and transfected into PANC-1, CAPAN-1, MIA-1, and A549 cells. Cell viability was compared after transfection with the RIP-tk genetic construct and daily treatment with of ganciclovir (GCV). RT-PCR was performed on PANC-1, CAPAN-1, and MIA-1 total RNA with primers specific for known insulin transcription factors PDX-1 and BETA-2. EMSA was also performed on PANG-1 and CAPAN-1 nuclear extract using an antibody specific to PDX-1. A mutated RIP-LacZ construct was created with one PDX-1 binding site chang...
example 3
Human Ductal Pancreatic Adenocarcinoma Cells that Express PDX-1 can be Targeted with a RIP-tk Gene
[0170]RIP-LacZ was created and transfected into CAPAN-1 (C-1) and MIA-1 (M−1) cell lines. RIP-tk was created and transfected into C-1 and M-1 cell lines. Tk driven by a ubiquitous promoter (U-tk), a hollow vector (HV) and untransfected cells (UT) were used as controls. Cells were treated for five days with 15 μg / ml of ganciclovir and cell viability was assessed during a MTS assay. RT-PCR was performed on C-1 and M-1 RNA with primers specific for PDX-1 and BETA-2. Nuclear extract from C-1 cells was subjected to a gel shift assay with an antibody to PDX-1. A PDX-1 binding site on RIP was mutated (mRIP) with PCR and a mRIP-LacZ construct was created and transfected in C-1 cells. Results are shown in Table 5.
TABLE 5Results: LacZ expression (LU)Percent Cell DeathRIPZRSVZmRIPZRIP-tkU-tkHVUTC-14.2 × 1059.1 × 1051.4 × 105T13 ± .1*8 ± .10 ± .14 ± .1M-11.2 × 1059.5 × 1050 ± .17 ± .10 ± .10 ± .1Ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com