Cardiac stem cell populations for repair of cardiac tissue
a stem cell and cardiac tissue technology, applied in the direction of skeletal/connective tissue cells, biocide, plant growth regulators, etc., can solve the problems of cardiac insufficiency progression, source and availability of sc remains, and permanent deficiency in the number of functioning cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
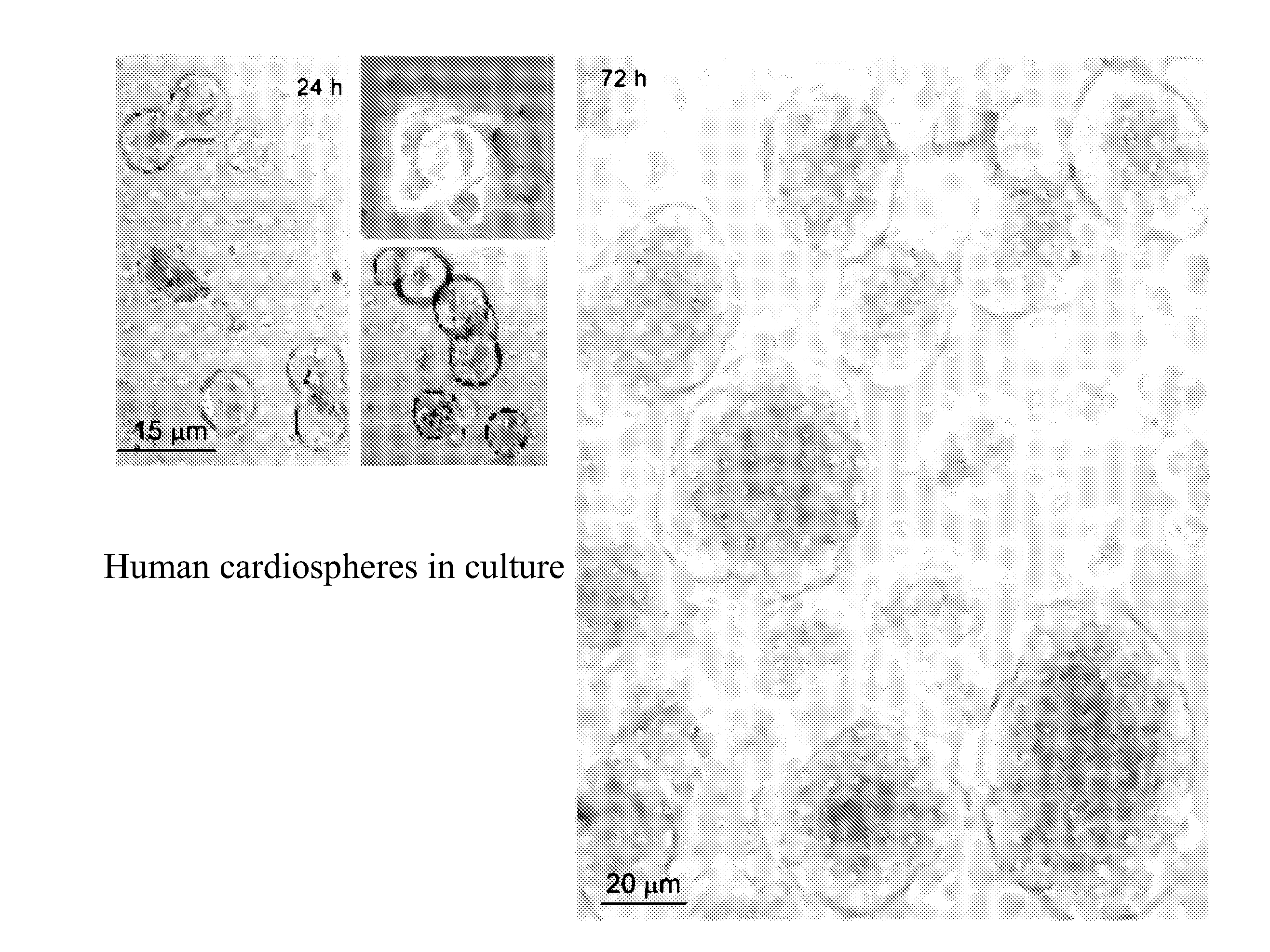
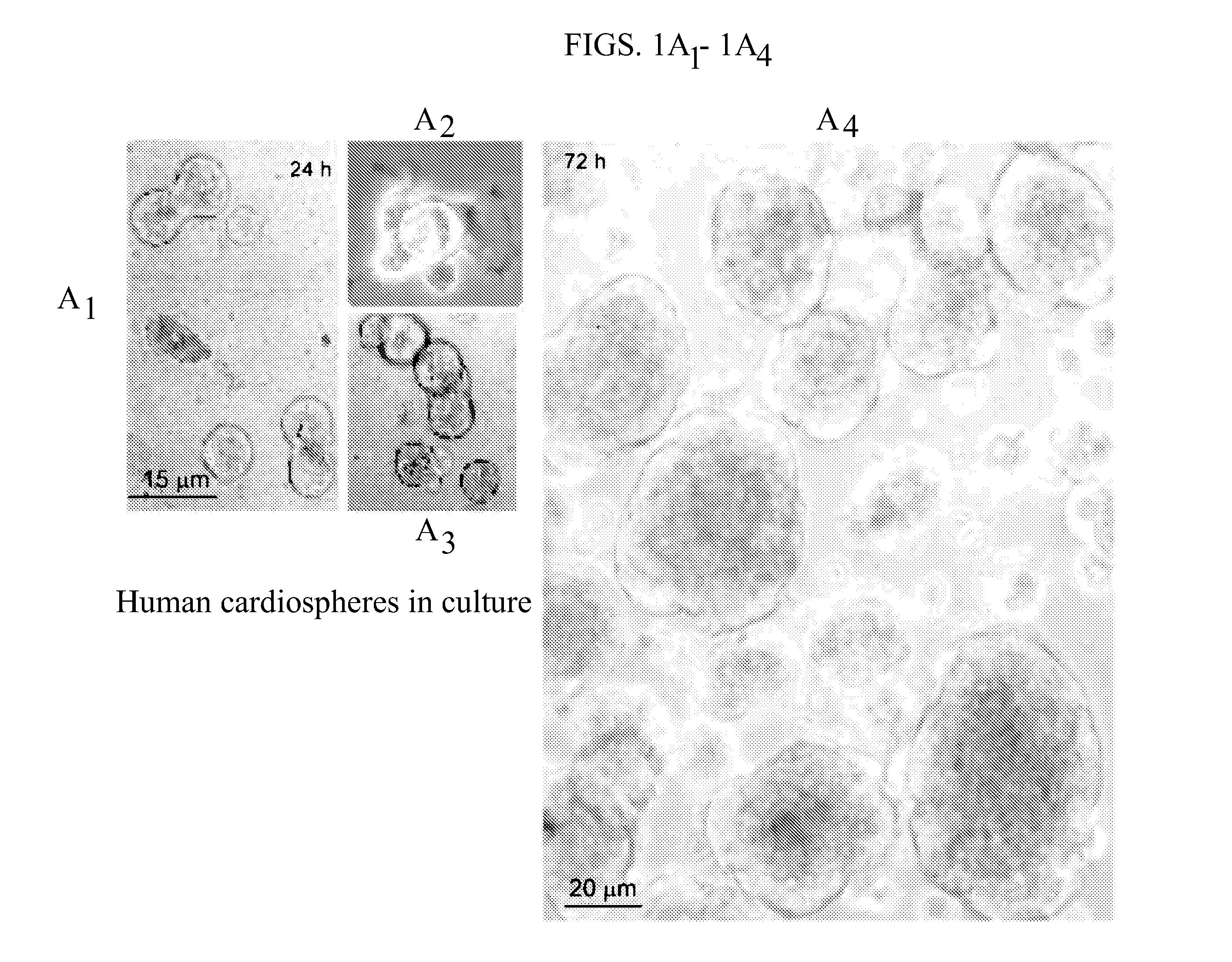
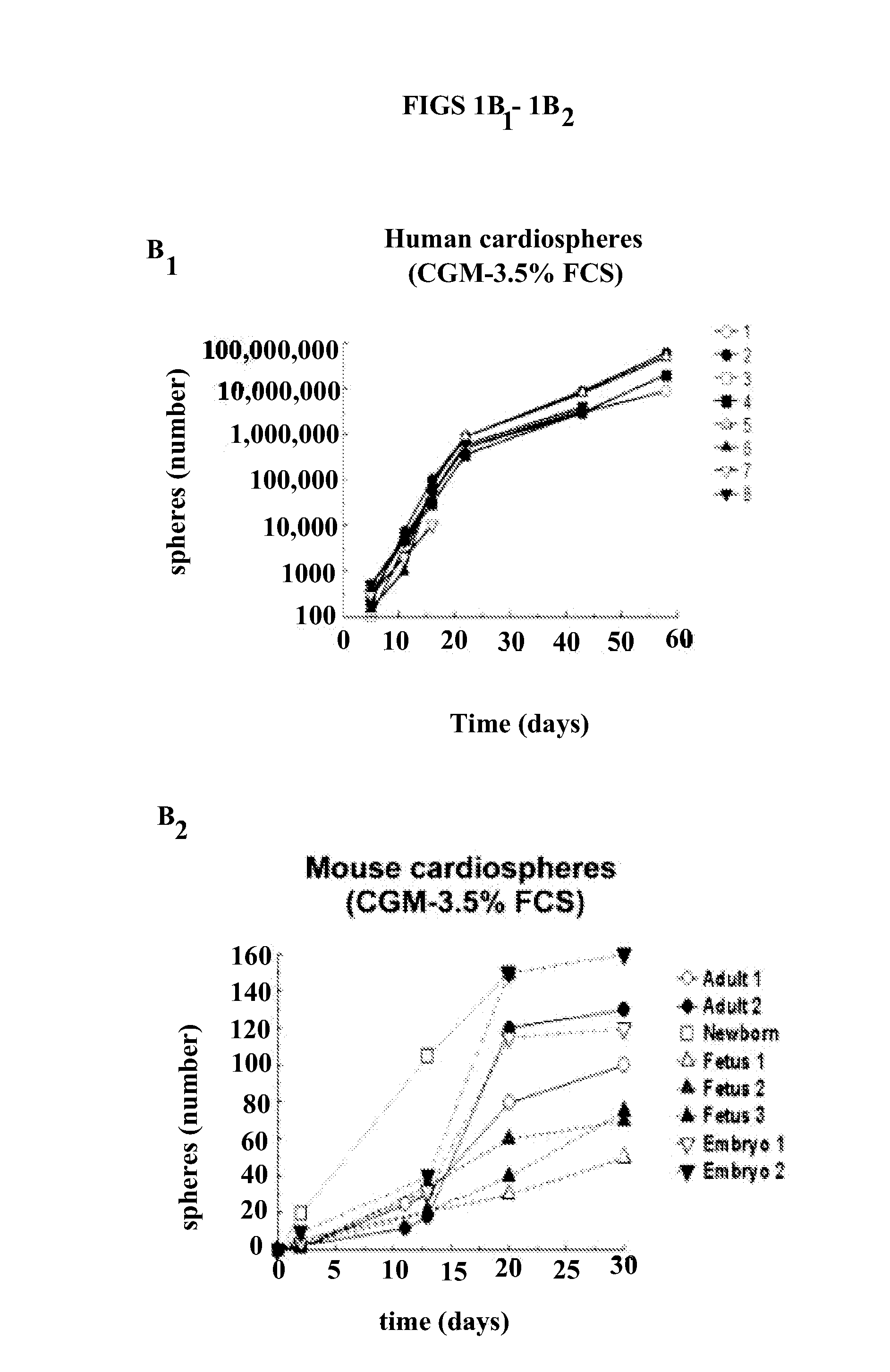
[0019]The method of the present invention employs heart biopsy tissue as starting material, hence an elective material that cannot be used in the method described in patent application WO 99 / 49015, since the material was insufficient. After fragmenting the biopsy specimen and possibly using dissociating agents (e.g. trypsin, EDTA and collagenase), the fragments are plated and added to a medium containing 10% FBS; 10-30 days later, fibroblast-like adherent cells grow from the explants over which small round, phase-bright cells migrate that tend to cluster but are either not or only weakly adherent. The cells are isolated by washing and mild dissociation (e.g. EDTA, trypsin-EDTA for 2-3 min). The cells are then plated on polylysine-treated cellular substrates in an appropriate medium unlike that used in the previous technique, in that it is horse-serum-free and contains other growth factors; after 2-3 days cell aggregates (cardiospheres) arise that tend to grow as floating formations....
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com